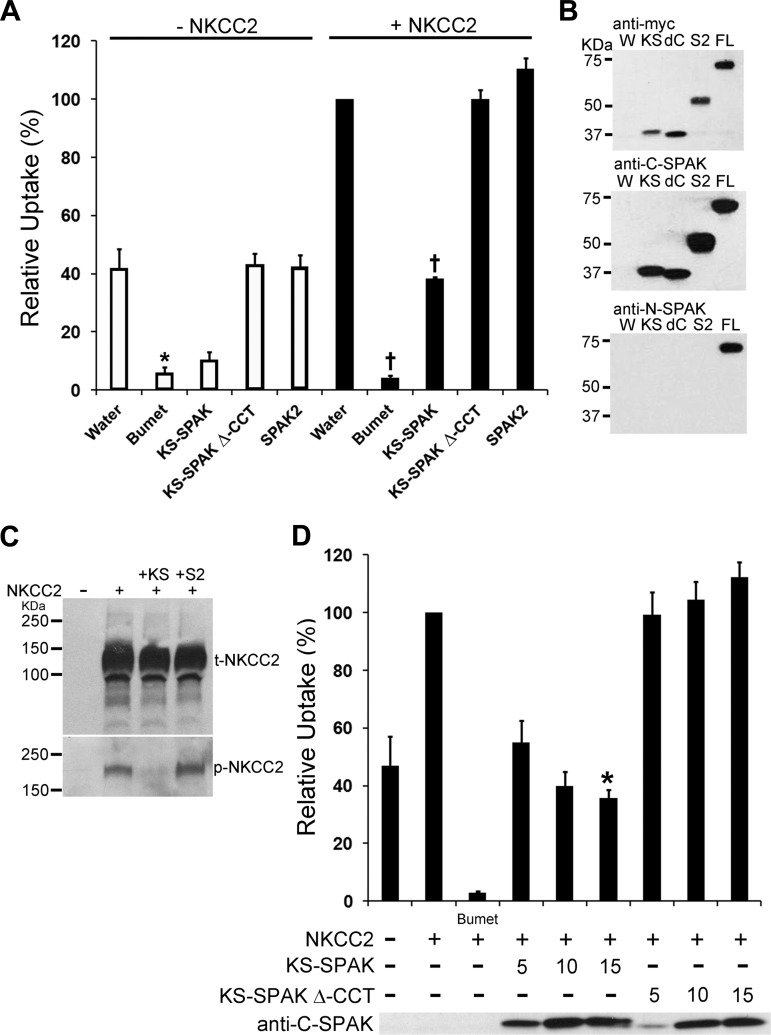
Fig. 1.
Kidney-specific (KS)-Ste20-related proline alanine-rich kinase (SPAK) inhibits cation cotransporter activity in Xenopus oocytes. A: 13–14 oocytes per group were injected with water or 10 ng of each capped RNA (cRNA) as shown. After 3 days 86Rb uptake was measured for pooled groups of oocytes under hypertonic (300 mosM) conditions. Data are expressed relative to water-injected oocytes (100%) ± SE. Water-injected oocytes treated with 1 mM bumetanide (Bumet) confirmed that the uptake in the water-injected group was through endogenous Na+-K+-2Cl− cotransporter 1 (NKCC1) or coinjected NKCC1 or NKCC2. Deletion of the conserved carboxy terminus (CCT), required for interactions with both cation cotransporters and with-no-lysine[K] (WNK) kinases, prevented inhibition of uptake by KS-SPAK (KS-SPAK Δ-CCT). *P < 0.001, compared with water injected; †P < 0.001, compared with NKCC2 only-injected; n = 4 (different frogs). B: Western blotting confirmed expression of the appropriate SPAK isoforms. W, water injected; KS, KS-SPAK; dC, KS-SPAK Δ-CCT; S2, SPAK2; FL, FL-SPAK. All isoforms have an NH2-terminal myc tag (top); each SPAK possesses the SPAK COOH terminus (middle). Anti-N-SPAK confirmed that only FL-SPAK has the SPAK NH2 terminus. C: immunoblotting showed that KS-SPAK, but not SPAK2, reduced phosphorylation of NKCC2 at the SPAK/OSR1 phosphorylation sites T95/T100 (human numbering), without an effect on total NKCC2 expression levels. D: titration of KS-SPAK revealed a dose-dependent inhibitory effect of KS-SPAK and confirmed the requirement for the Δ-CCT. *P = 0.05, compared with 5 ng KS-SPAK; n = 4 (different frogs).