Fig. 4.
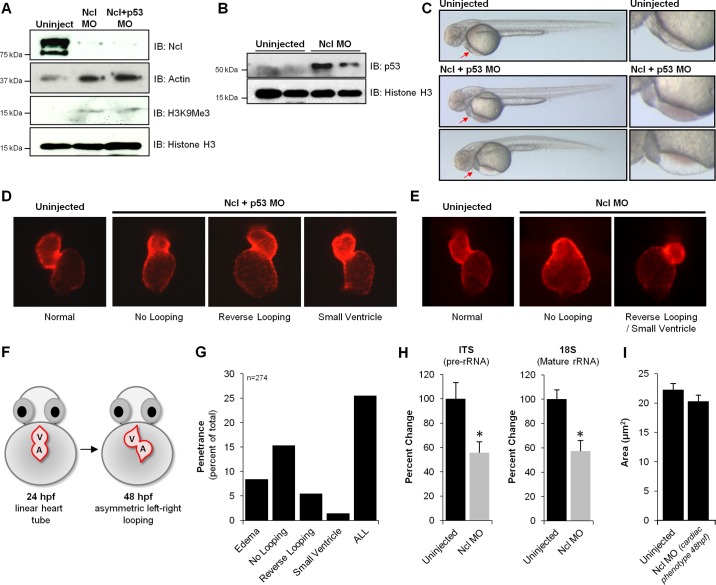
Loss of nucleolin in zebrafish results in cardiac morphological defects. To examine the role of Nucleolin on zebrafish development, Morpholino-based knockdown was performed. Embryos were dechorinated and injected at the one cell stage with 3 ng of Nucleolin (Ncl) Mopholino (MO) in the presence or absence of p53 MO (3 ng). Note: in this experiment, the coinjection with p53 is a control to rule out generalized cell death phenotypes induced by the Nucleolin MO; in all experiments performed, the coinjected Ncl+p53 was indistinguishable from the Ncl alone, and so the former is shown as this is the more rigorous control. A: Western blot analysis of 24 hpf embryos reveals near complete loss of Nucleolin protein, concomitant with significant increases in Histone H3 trimethylation on lysine K9 (H3K9Me3) and p53 (B), supporting an endogenous role for Nucleolin to maintain euchromatin (Actin and total Histone H3 shown as controls). C: depletion of Nucleolin protein had minimal effects on gross development in zebrafish, as observed 48 hpf (a time point at which Nucleolin protein levels are still significantly diminished), with the striking exception of the heart, in which a significant population of embryos displayed blood pooling (middle panels) and/or edema (fluid accumulation in pericardial space; bottom panels), both symptoms of cardiac dysfunction. Red arrows highlight regions of defect, expanded in right column. To further investigate this localized cardiac phenotype, we examined heart development after Ncl KD in the presence (D) or absence (E) of p53 MO by fluorescence imaging. Embryos expressing mCherry fluorescent protein under control of the cmlc2 promoter enabled live imaging of heart morphology (images taken at 48 hpf). F: normal cardiac development involves looping of the linear heart tube between 24 and 36 hpf, eventually exhibiting proper orientation by 48 hpf; however, three prominent defects in chamber patterning and morphology were observed after Nucleolin knockdown: no looping, reverse looping and decreased ventricular size (small ventricle). G: penetrance of phenotypes observed after Nucleolin knockdown indicates that ∼25% of zebrafish displayed a cardiac defect, with delayed looping being the most prominent phenotype (15%). H: to investigate the mechanistic basis for these cardiac defects, rRNA transcription and maturation was examined using primers that detect pre-rRNA (ITS) and mature rRNA (18S) by qRT-PCR, demonstrating that loss of Nucleolin impaired both rRNA transcription and processing (n = 3/group). Asterisks indicate a P value < 0.01. I: embryos expressing cardiac restricted mCherry fluorescent protein (red) 72 hpf were fixed and stained via whole mount immunohistochemistry against Zn8, a cell membrane marker (green). Confocal microscopy was used to optically section the heart and generate a 2-dimensional maximum intensity projection of several images acquired serially in the z-dimension. Zn8 staining in ventricular cardiomyocytes enabled quantification of cell size in embryos with normal or reduced Nucleolin levels (displaying a cardiac phenotype at 48 hpf).