Abstract
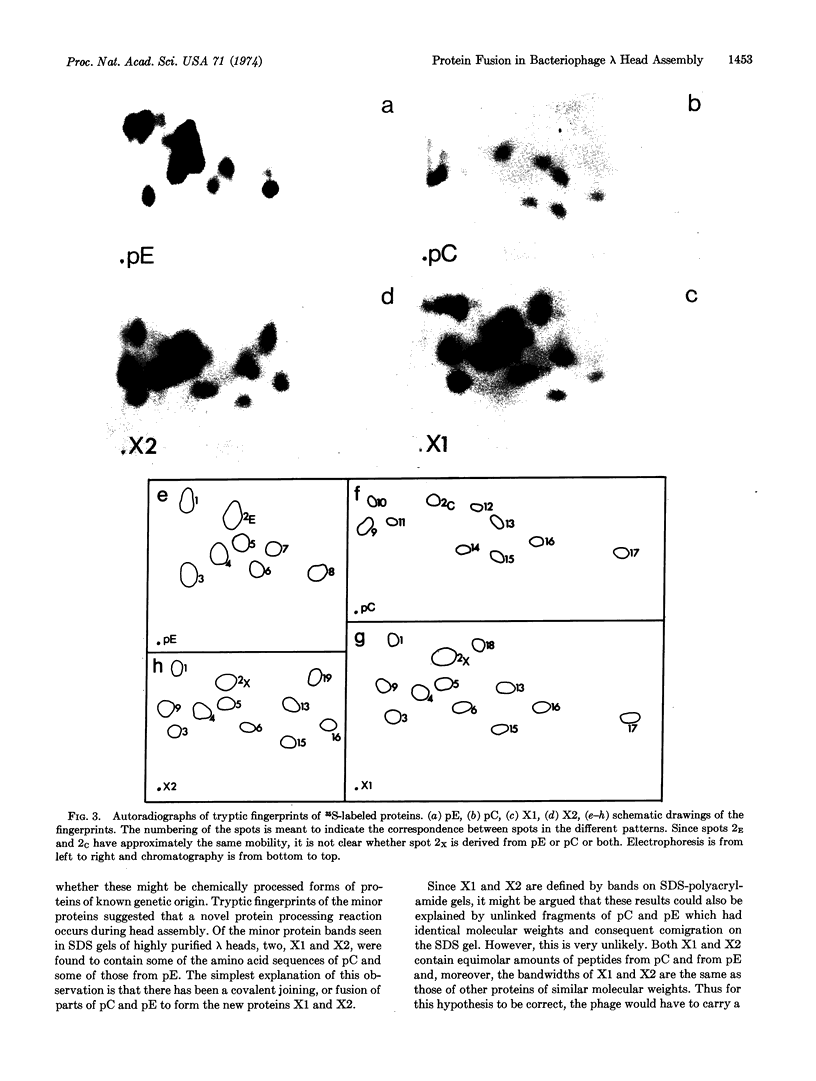
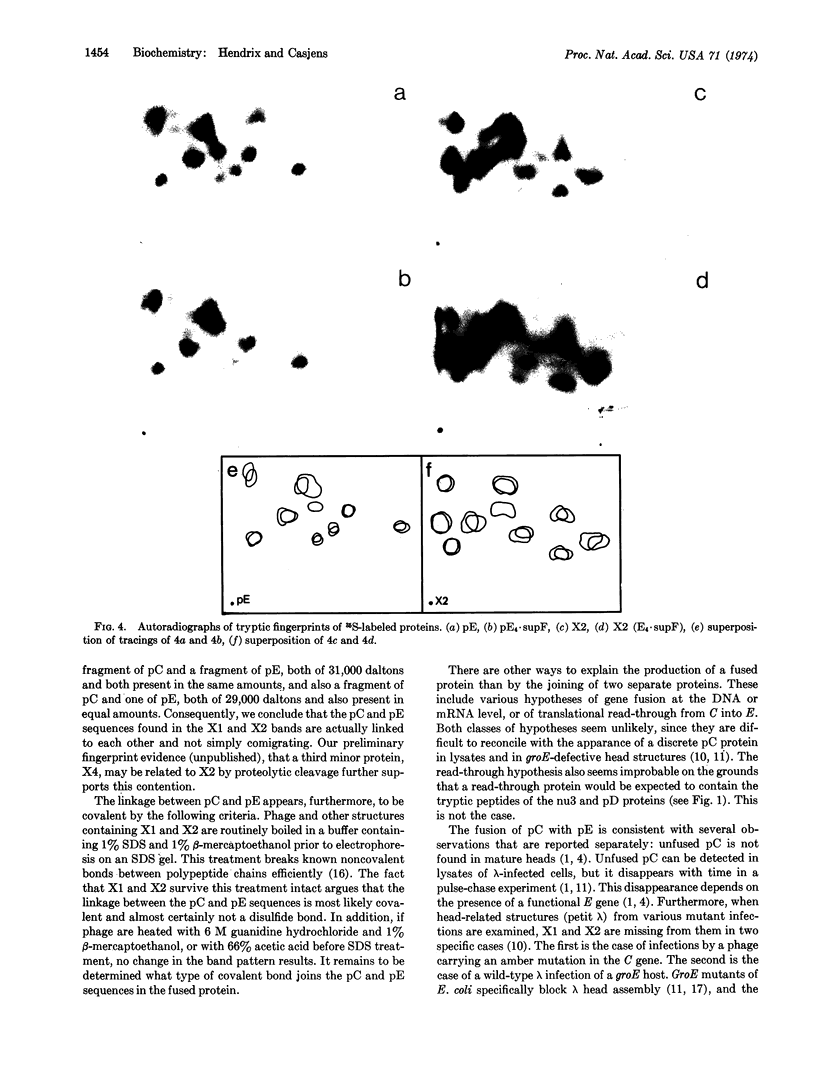
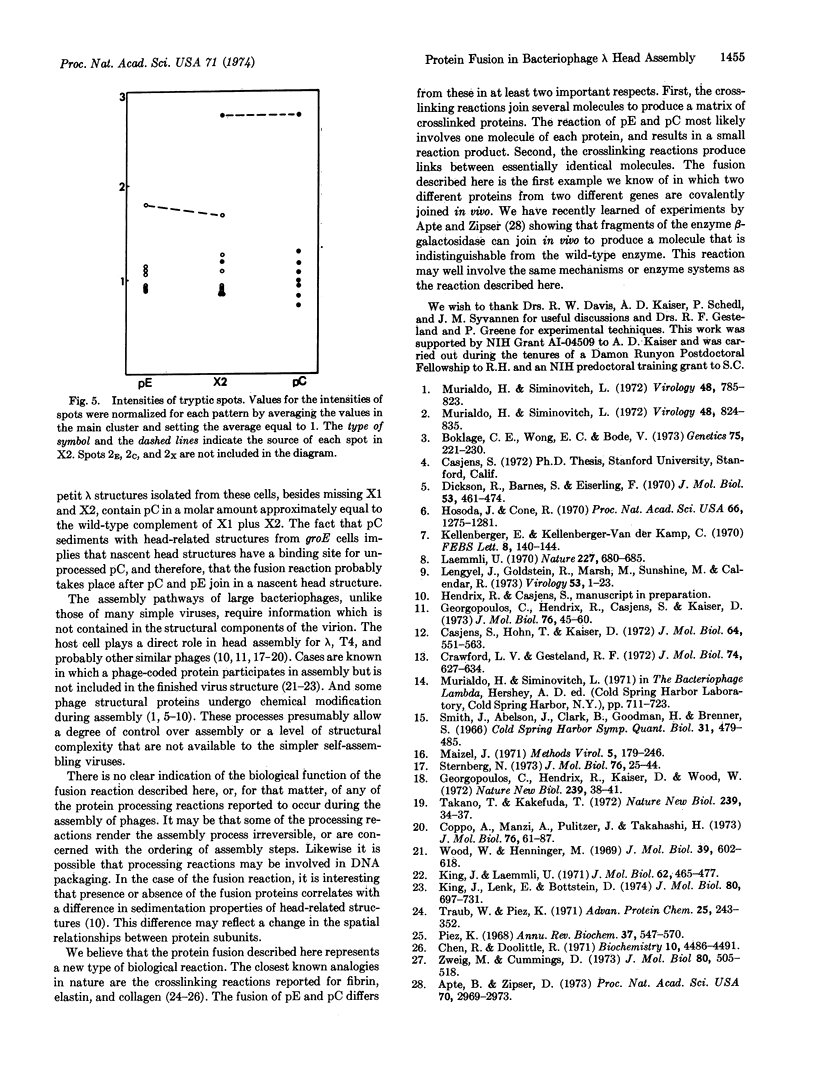
Parts of two phage-coded head proteins, pE and pC, become fused during bacteriophage λ head assembly. pE is the main structural component of λ heads and pC is a minor head protein that is not found as such in mature heads. The bond joining the two proteins appears to be covalent and is not a disulfide bond. Only a specific subset of the sequences of each protein is found in the fusion products, and these sequences are found in the products in equimolar amounts. Two nearly identical fusion products; X1 and X2, are detected; X2 is slightly smaller than X1 and appears to be a proteolytic cleavage product of X1. The fusion reaction probably takes place on a nascent head structure.
Keywords: protein processing, viral morphogenesis, protein splicing
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte B. N., Zipser D. In vivo splicing of protein: one continuous polypeptide from two independently functioning operons. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2969–2973. doi: 10.1073/pnas.70.10.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklage C. E., Wong E. C., Bode V. C. The lambda F mutants belong to two cistrons. Genetics. 1973 Oct;75(2):221–230. doi: 10.1093/genetics/75.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Horn T., Kaiser A. D. Head assembly steps controlled by genes F and W in bacteriophage lambda. J Mol Biol. 1972 Mar 14;64(3):551–563. doi: 10.1016/0022-2836(72)90082-4. [DOI] [PubMed] [Google Scholar]
- Chen R., Doolittle R. F. - cross-linking sites in human and bovine fibrin. Biochemistry. 1971 Nov 23;10(24):4487–4491. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. IV. Identification of gene products and control of the expression of the morphogenetic information. Virology. 1972 Jun;48(3):785–823. doi: 10.1016/0042-6822(72)90162-6. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of phage lambda. V. Form-determining function of the genes required for the assembly of the head. Virology. 1972 Jun;48(3):824–835. doi: 10.1016/0042-6822(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Abelson J. N., Clark B. F., Goodman H. M., Brenner S. Studies on amber suppressor tRNA. Cold Spring Harb Symp Quant Biol. 1966;31:479–485. doi: 10.1101/sqb.1966.031.01.062. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]