Figure 4.
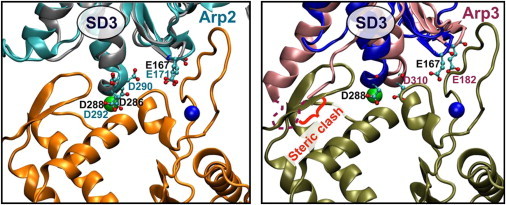
Conserved cation-binding residues between Arp2/3 and actin suggest salt-dependent regulation of Arp2/3 activation. Arp2 and Arp3 are overlaid with an actin subunit (best alignment calculated by the software FATCAT (88)) and interact with the next longitudinally neighboring subunit at the barbed-end face of Arp2/3. Actin subunits at the bottom of each panel represent the first two actin monomers that associate with Arp2/3 to nucleate the daughter filament at a branch point. Arp2 aligns very well with actin, especially the putative cation-binding residues E171, D290, and D292, which help form the polymerization and stiffness cation-binding sites with the incoming actin monomer. Arp3 does not align as well in the inactive crystal conformation. Arp2/3 activation is thought to require WASP/Scar-dependent conformational rearrangement of Arp3. We hypothesize that this rearrangement relieves a steric clash with the incoming actin monomer while forming a better cation-binding geometry at both the polymerization and stiffness cation-binding sites shared with the incoming daughter filament subunit. In this figure, pivoting of the Arp3 SD3 to the left would both alleviate the steric clash and place E182 and D310 into the proper position to bind interfacial cations. To see this figure in color, go online.
