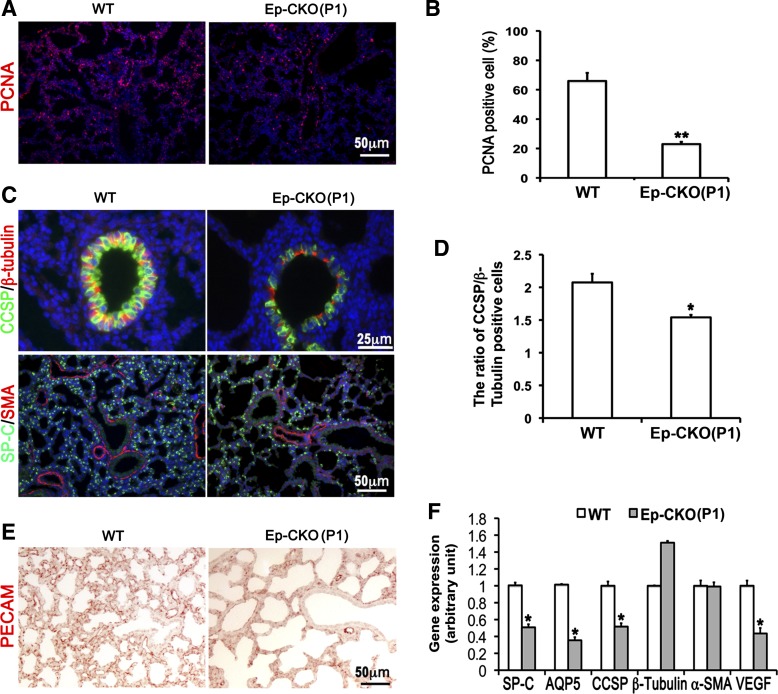
Fig. 4.
Lung epithelium-specific deletion of TACE resulted in decreased cell proliferation, impaired epithelial cell differentiation, and reduced pulmonary capillary formation. A and B: cell proliferation was detected by proliferating cell nuclear antigen (PCNA) immunostaining and analyzed as a percentage of PCNA-positive cells. Cell proliferation was significantly reduced in P1 TACE Ep-CKO lung (23.0 ± 1.5%, **P < 0.01) compared with wild-type controls (66.0 ± 5.5%). C and D: immunostaining of molecular markers of differentiated lung cells in TACE Ep-CKO lungs. Alveolar epithelial cells were significantly reduced in the P1 TACE Ep-CKO lung. Moreover, club cells in the airways were also significantly reduced and flattened in shape in TACE Ep-CKO compared with the controls. The reduction of club cell in bronchiole was quantified by calculating the ratio of club cell-specific protein (CCSP)-positive to β-tubulin IV-positive cells (2.08 ± 0.13 in wild-type controls vs. 1.54 ± 0.13 in Ep-CKO lung, *P = 0.013). Expression level and distribution pattern of α-smooth muscle actin (SMA), a marker of smooth muscle cells and myofibroblasts, were similar between TACE Ep-CKO and normal control lungs. Cell nuclei were counterstained by DAPI (blue). E: reduced platelet endothelial cell adhesion molecule 1 (PECAM)-positive cells demonstrate impaired capillary formation in Ep-CKO lung. F: gene expression of surfactant protein C (SP-C), aquaporin 5 (AQP5), CCSP, and vascular endothelial growth factor α (VEGFα) at the mRNA level was significantly decreased in TACE Ep-CKO lung as measured by real-time PCR. *P < 0.05.