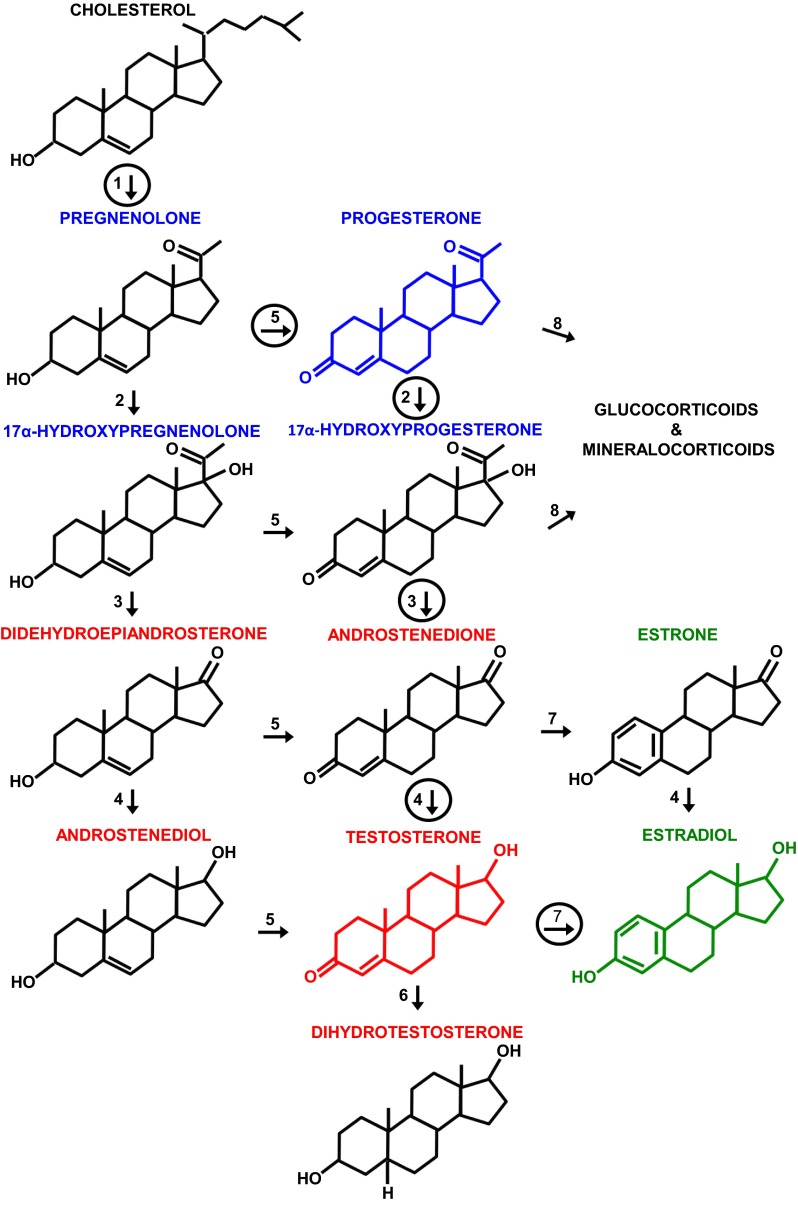
Fig. 1.
The principal pathways of human gonadal steroid hormone synthesis. Molecules are shown in standard line-angle diagrams, and enzymes are represented as numbered arrows, with the major pathway in adult gonads circled. Steroidogenesis begins with the cleavage of the 6 C side chain from cholesterol (C27H46O) by the mitochondrial cholesterol side-chain cleavage enzyme, otherwise known as P-450scc or CYP11A1 (arrow 1) to yield pregnenolone (C21H32O2). Note that it and other progestins (or progestagens; labeled in blue, with the structure of progesterone, the principal progestin, also in blue) are 21 C molecules. Subsequent steps occur on the smooth endoplasmic reticulum. Progestins are metabolized to androgens (labeled in red, with the principal androgen, testosterone, diagrammed in red), which are 19 C, and to mineralocorticoids and glucocorticoids (not shown), which are 21 C. Androgens are metabolized to estrogens (labeled in green, with the principal estrogen, estradiol, diagrammed in green), which are 18 C. Note that all of these steroids retain the basic 17 C “gonane” structure, consisting of three cyclohexane rings and one cyclopentane ring, but differ in the attached side groups and oxidation states of the rings. An additional estrogen, estriol (not shown), is synthesized in significant amounts only by the placenta and fetal liver. Other labeled enzymes: 2, 17α-hydroxylase; 3, 17, 20-lyase; 4, 17β-hydroxysteroid dehydrogenase;5, 3β-hydroxysteroid dehydrogenase; 6, 5α-reductase; 7, aromatase; 8, 21-hydroxylase.