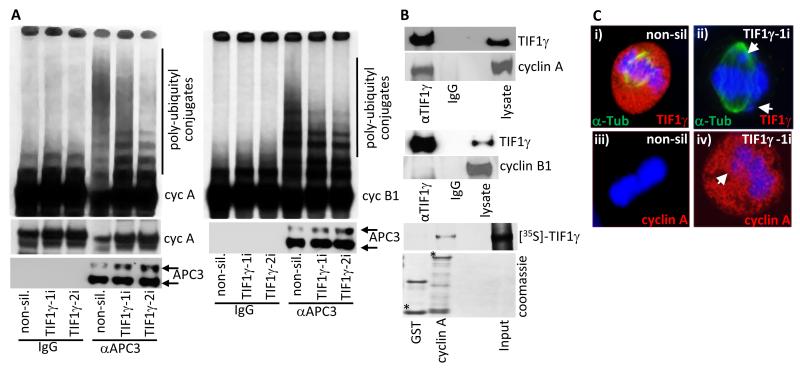
Figure 6. Cyclin A is stabilized in mitotic TIF1γ knockdown cells and is degraded upon expression of an siRNA resistant TIF1γ species.
(A) APC/C ligase activity directed towards cyclin A and cyclin B1 is reduced following TIF1γ knockdown. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ. The APC/C holoenzyme was immunoprecipitated from cell lysates 72h post-treatment using an antibody to APC3. APC/C activity was then assayed by incubating anti-APC3 immunoprecipitates with L-α-[35S]-methionine labeled cyclin A or cyclin B1. After 1 h incubation samples were separated by SDS-PAGE and polyubiquitylation of cyclin A and cyclin B1 was assessed following autoradiography of the dried gel. (B) TIF1γ associates with cyclin A but not cyclin B1. HeLa cells were subject to immunoprecipitation with TIF1γ, and Western blotted for cyclin A or cyclin B1; GST and GST-cyclin A were incubated with L-α-[35S]-methionine labelled TIF1γ GST- fusion, and binding proteins were purified upon glutathione-agarose beads and separated by SDS-PAGE. The binding of TIF1γ to GST-cyclin A was assessed following autoradiography of the dried gel. * indicates full-length GST fusion protein; upper band in GST lane is GST dimer. (C) Cyclin A is inappropriately present at metaphase in TIF1γ knockdown cells. HeLa cells were treated with either non-silencing siRNA or siRNAs specific for TIF1γ and seeded onto glass slides. 72h post-transfection cells were fixed and permeabilized and then co-stained with either α-tubulin, TIF1γ and DAPI (i) or cyclin A and DAPI (ii)