Abstract
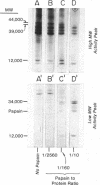
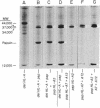
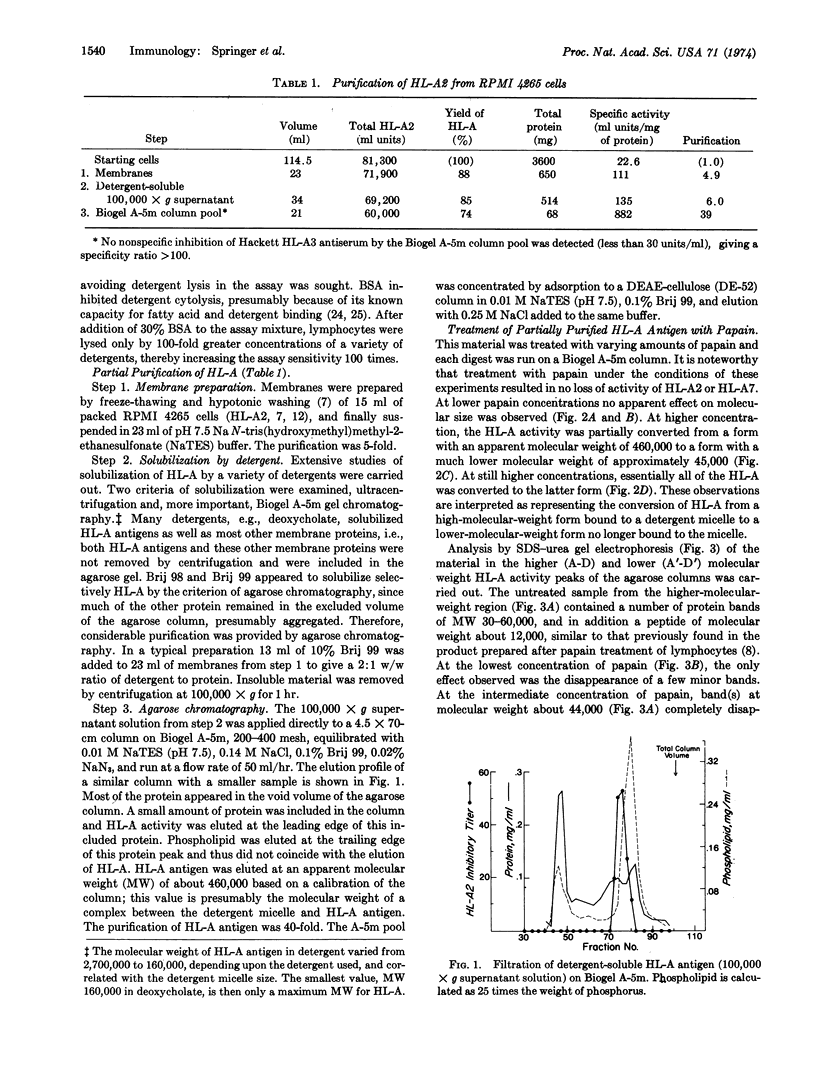
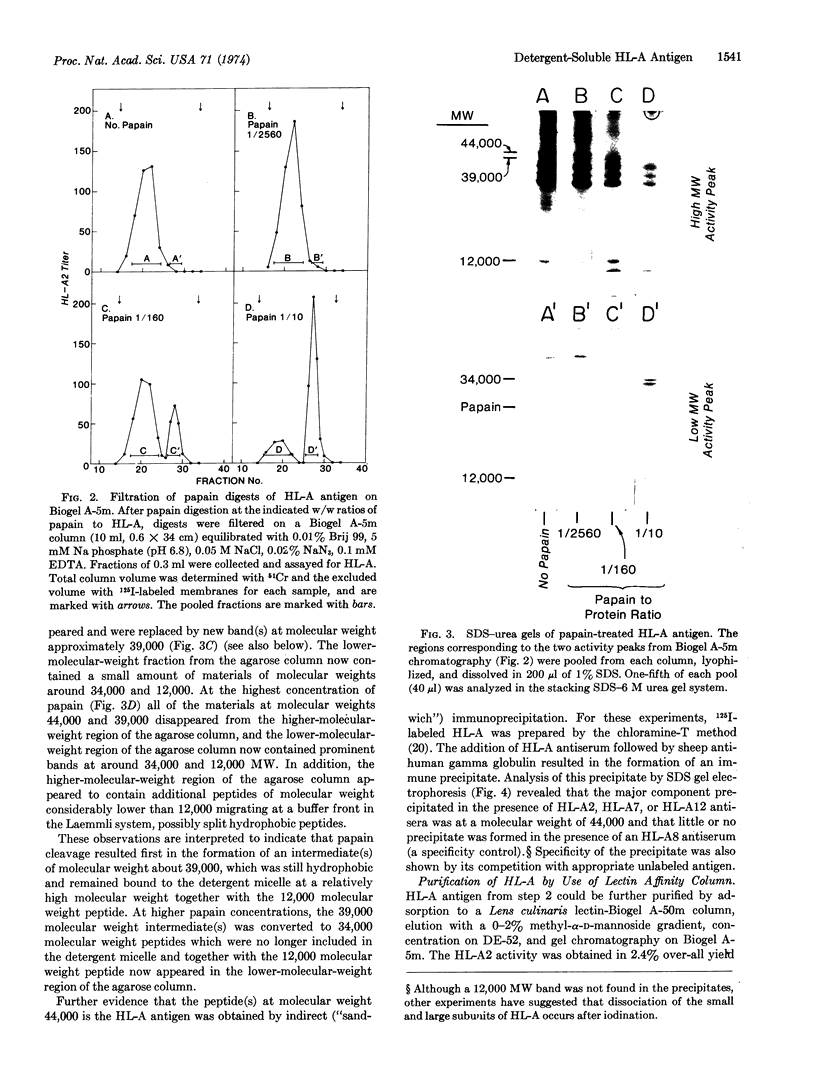
HL-A antigen solubilized with the non-ionic detergent, Brij 99, has been purified to about 50% of homogeneity from a cultured human lymphoblast line. It consists of two nonidentical subunits of 44,000 and 12,000 molecular weight (MW). Upon papain proteolysis the 44,000 MW peptide is converted by at least two cleavages to a 34,000 MW peptide, but the 12,000 MW peptide appears to be unchanged. Concomitantly, the apparent molecular weight in gel filtration chromatography under nondenaturing conditions in the presence of Brij 99 is reduced from 460,000 to 45,000. HL-A molecules produced by direct papain proteolysis of membranes and by papain treatment of purified detergent-soluble HL-A are identical.
Keywords: β2-microglobulin, Brij 99, histocompatibility
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Cresswell P., Turner M. J., Strominger J. L. Papain-solubilized HL-A antigens from cultured human lymphocytes contain two peptide fragments. Proc Natl Acad Sci U S A. 1973 May;70(5):1603–1607. doi: 10.1073/pnas.70.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautigny A., Bernier I., Colombani J., Jollés P. Purification and characterization of HL-A antigens from human platelets, solubilized by the non-ionic detergent NP-40. Biochim Biophys Acta. 1973 Apr 16;298(4):783–789. doi: 10.1016/0005-2736(73)90382-9. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Silver J., Sheppard L. B., Amos D. B. The purification of detergent-solubilized HL-A antigens by affinity chromatography with the hemagglutinin from Lens culinaris. J Immunol. 1974 Mar;112(3):1190–1193. [PubMed] [Google Scholar]
- Eijsvoogel V. P., Du Bois R., Melief C. J., Zeylemaker W. P., Raat-Koning L., de Groot-Kooy L. Lymphocyte activation and destruction in vitro in relation to MLC and HL-A. Transplant Proc. 1973 Mar;5(1):415–420. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Hilgert I., Kandutsch A. A., Cherry M., Snell G. D. Fractionation of murine H-2 antigens with the use of detergents. Transplantation. 1969 Oct;8(4):451–461. doi: 10.1097/00007890-196910000-00016. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine B. B., Stember R. H., Fotino M. Ragweed hay fever: genetic control and linkage to HL-A haplotypes. Science. 1972 Dec 15;178(4066):1201–1203. doi: 10.1126/science.178.4066.1201. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Rogentine G. N., Fahey J. L., Nathenson S. Human lymphocyte membrane (HL-A) alloantigens: isolation, purification and properties. J Immunol. 1969 Aug;103(2):282–292. [PubMed] [Google Scholar]
- Mann D. L., Rogentine G. N., Jr, Fahey J. L., Nathenson S. G. Molecular heterogeneity of human lymphoid (HL-A) alloantigens. Science. 1969 Mar 28;163(3874):1460–1462. doi: 10.1126/science.163.3874.1460. [DOI] [PubMed] [Google Scholar]
- Mann D. L. The effect of enzyme inhibitors on the solubilization of HL-A antigens with 3 M KCl. Transplantation. 1972 Sep;14(3):398–401. [PubMed] [Google Scholar]
- Miyakawa Y., Tanigaki N., Kreiter V. P., Moore G. E., Pressman D. Characterization of soluble substances in plasma carrying HL-A alloantigenic activity and HL-A common antigenic activity. Transplantation. 1973 Mar;15(3):312–319. doi: 10.1097/00007890-197303000-00008. [DOI] [PubMed] [Google Scholar]
- Nakamuro K., Tanigaki N., Pressman D. Multiple common properties of human beta2-microglobulin and the common portion fragment derived from HL-A antigen molecules. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2863–2865. doi: 10.1073/pnas.70.10.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G. Biochemical properties of histocompatibility antigens. Annu Rev Genet. 1970;4(0):69–90. doi: 10.1146/annurev.ge.04.120170.000441. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J Clin Invest. 1957 Apr;36(4):538–542. doi: 10.1172/JCI103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Pellegrino M. A., Kahan B. D. Salt extraction of soluble HL-A antigens. Science. 1971 Jun 11;172(3988):1134–1136. doi: 10.1126/science.172.3988.1134. [DOI] [PubMed] [Google Scholar]
- SANDERSON A. R. CYTOTOXIC REACTIONS OF MOUSE ISO-ANTISERA: PRELIMINARY CONSIDERATIONS. Br J Exp Pathol. 1964 Aug;45:398–408. [PMC free article] [PubMed] [Google Scholar]
- Sanderson A. R. HL-A substances from human spleens. Nature. 1968 Oct 12;220(5163):192–195. doi: 10.1038/220192a0. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Kato K., Cullen S. E., Nathenson S. G. H-2 histocompatibility alloantigens. Some biochemical properties of the molecules solubilized by NP-40 detergent. Biochemistry. 1973 May 22;12(11):2157–2164. doi: 10.1021/bi00735a023. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Shimada A., Nathenson S. G. Murine histocompatibility-2 (H-2) alloantigens. Purification and some chemical properties of soluble products from H-2b and H-2d genotypes released by papain digestion of membrane fractions. Biochemistry. 1969 Oct;8(10):4048–4062. doi: 10.1021/bi00838a023. [DOI] [PubMed] [Google Scholar]
- Snary D., Goodfellow P., Hayman M. J., Bodmer W. F., Crumpton M. J. Subcellular separation and molecular nature of human histocompatibility antigens (HL-A). Nature. 1974 Feb 15;247(5441):457–461. doi: 10.1038/247457a0. [DOI] [PubMed] [Google Scholar]
- Tichá M., Entlicher G., Kostír J. V., Kocourek J. Studies on phytohemagglutinins. IV. Isolation and characterization of a hemagglutinin from the lentil, Lens, esculenta, Moench. Biochim Biophys Acta. 1970 Nov 17;221(2):282–289. doi: 10.1016/0005-2795(70)90268-0. [DOI] [PubMed] [Google Scholar]
- Utsumi S. Stepwise cleavage of rabbit immunoglobulin G by papain and isolation of four types of biologically active Fc fragments. Biochem J. 1969 Apr;112(3):343–355. doi: 10.1042/bj1120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Fudenberg H. H. Fc and Fab fragments from IgG 2 human immunoglobulins characterized. Nat New Biol. 1972 Nov 1;240(96):24–26. doi: 10.1038/newbio240024a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yunis E. J., Amos D. B. Three closely linked genetic systems relevant to transplantation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3031–3035. doi: 10.1073/pnas.68.12.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]