Figure 4.
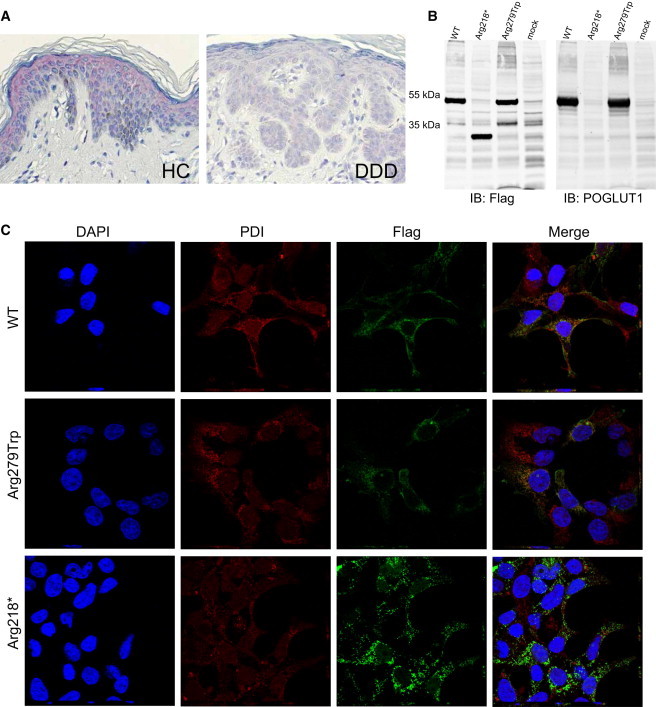
Immunohistochemistry on Skin Biopsies, Immunoblotting, and Immunofluorescence Analysis of HEK293T cells Transiently Expressing Wild-Type and Mutant POGLUT1
(A) For immunohistochemistry, sections were prepared from formalin-fixed, paraffin-embedded skin biopsies obtained by plastic surgery from individuals with DDD and healthy controls. POGLUT1 localization was analyzed with the polyclonal antibody NBP1-90311 (named KTELC1; Novus Biologicals) at a 1:500 dilution. Visualization was performed with the LSAB2 staining kit (DAKO) with Fast Red Chromogen.
POGLUT1 was strongly present in the epidermis of healthy controls, especially in the upper parts (the stratum spinosum and stratum granulosum). POGLUT1 staining was weaker in lesional skin of individuals affected by DDD.
(B) For immunoblotting, WT sequence, sequence bearing mutation c.652C>T (p.Arg218∗), and sequence bearing mutation c.835C>T (p.Arg279Trp) were cloned into the eukaryotic expression vector pAAV-CMV-MCS (Stratagene). HEK293T cells (European Collection of Cell Cultures [ECACC]) were transiently transfected with the plasmids by the use of Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hr after transfection, cells were lysed in ice cold lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1% Triton X-100) supplemented with proteinase inhibitors (Roche) for 30 min on ice followed by sonication and centrifugation at 14.000 rpm/10 min/4°C. Clear supernatant was boiled with SDS-sample buffer at 95°C for 5 min and the proteins were subjected to gel electrophoresis (SDS-PAGE, 10%) followed by transfer to nitrocellulose membrane (Millipore). Immunoblotting was performed with mouse anti-Flag (1804; Sigma Aldrich, 1:1.000), rabbit anti-POGLUT1 (NBP1-90311; Novus Biologicals, 1:1.000) primary antibodies and IRDye secondary antibodies (IRDye 800 goat anti-mouse and IRDye 680 goat anti-rabbit, 1:10.000). Bands of the expected size were detected for the WT POGLUT1 and the protein with the p.Arg279Trp substitution, whereas the nonsense mutation (c.652C>T [p.Arg218∗]) led to translation of a truncated protein of around 30 kDa.
(C) Immunofluorescence analysis was performed with transiently transfected HEK293T cells. Cells were fixed and incubated for 12–14 hr with mouse anti-Flag (1:400, Sigma-Aldrich) and rabbit anti-PDI (1:200, Abcam) antibodies. After several washing steps, cells were incubated for 40 min with Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 568 goat anti-mouse (1:300, Invitrogen), and DAPI (Invitrogen).
Images were acquired at room temperature with a laser-scanning confocal microscope (Nikon A1/Ti, Nikon) with a CFI Plan Apochromat infrared 60× water-immersion objective (NA 1.27). For each construct z stack (step: 0.250 um), images were taken with the NIS-Elements 4.0 acquisition software (Nikon).
The analysis revealed the colocalization of the WT POGLUT1 with endoplasmic reticulum (ER). No significant difference was observed in localization patterns of the WT protein and the protein with the amino acid substitution. However, a more aggregated pattern was observed for the truncated protein in comparison to the WT protein, which coincided with an impaired colocalization with the ER. Abbreviations are as follows: HC, healthy control; DDD, Dowling-Degos disease, IB, immunoblot.
