Fig. 2.
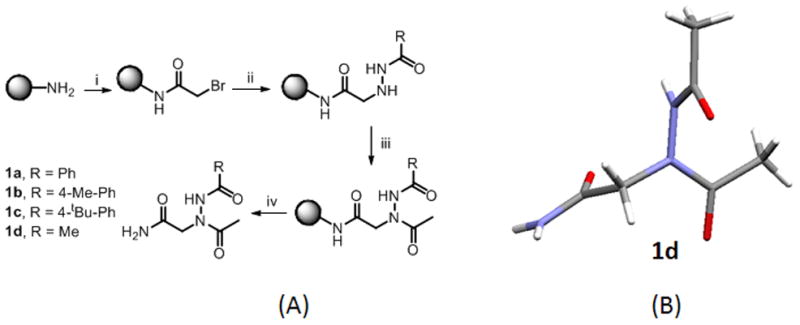
(A) Proposed sub-monomer synthesis of N-azapeptoids 1a–1d. For 100 mg resin (i) Bromoacetic acid (2M, 1mL) and DIC (3.4 M, 1mL), 37 ºC, 10 mins; (ii) Acyl hydrazide (2M, 2mL), 37 ºC, 1h (iii) acetic acid (2M, 1mL), DIC (3.4M, 1mL), 30 mins (iv) TFA:TIS:water (96:2:2) (2mL). (B) Single crystal X-ray structure of 1d shows the trans-amide geometry of both the main chain and side chain amide bonds.
