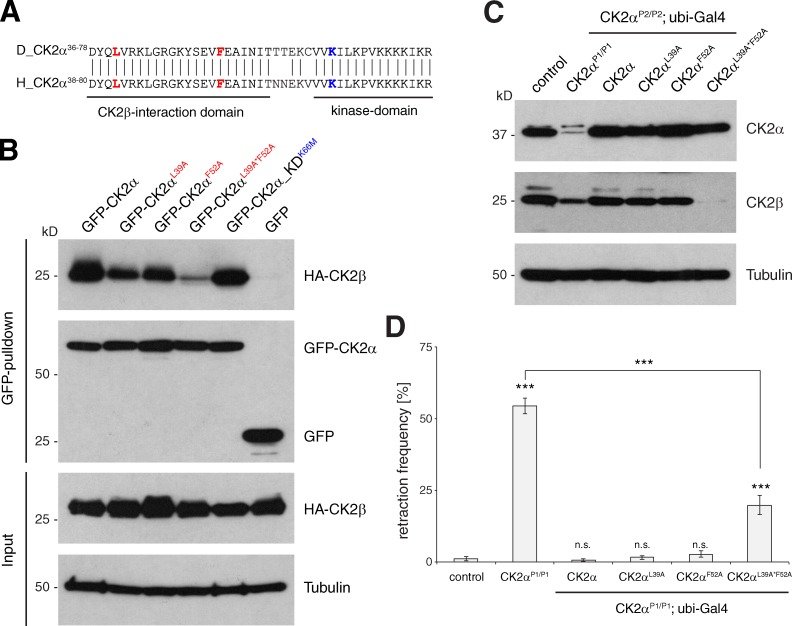
Figure 4.
CK2α–CK2β interaction is essential for the control of synapse stability. (A) Sequence comparison of Drosophila and human CK2α. Residues required for the CK2α–CK2β interaction (leucine 39 and phenylalanine 52 in Drosophila) are marked in red. Lysine 66 that is essential for CK2α kinase function is marked in blue. (B) S2 cell IP assay analyzing the CK2α–CK2β interaction. HA-tagged CK2β was efficiently precipitated by GFP-tagged wild-type or kinase-dead CK2α. The mutations CK2αL39A and CK2αF52A reduced the efficiency of the IP. The double mutant CK2αL39A*F52A caused a further reduction of the interaction. No pull-down was observed in the GFP control. (C) Western blot analysis of larval brain extracts demonstrated that ubiquitous expression of wild-type CK2α, CK2αL39A, or CK2αF52A in CK2αP2/P2-null animals efficiently restored CK2α and CK2β protein levels. In contrast, expression of CK2αL39A*F52A restored CK2α levels, but failed to rescue the reduction in CK2β protein levels. (D) Quantification of synaptic retraction frequencies (***, P ≤ 0.001, n = 12–17 animals, muscles 1/9 and 2/10). n.s., not significant. Error bars represent SEM.