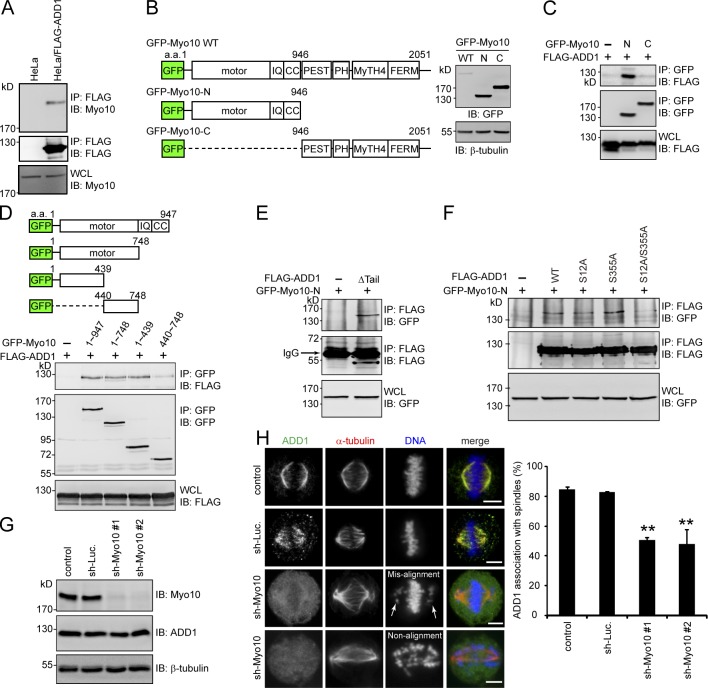
Figure 3.
ADD1 phosphorylation at S12 and S355 is required for its interaction with the motor domain of Myo10. (A) The cell lysates prepared from HeLa cells or those stably expressing FLAG-ADD1 were incubated with anti-FLAG M2 affinity resins. The bound proteins were eluted from the resins with FLAG peptides and analyzed by immunoblotting (IB) with anti-Myo10 or anti-FLAG. WCL, whole-cell lysates. (B) GFP-fused Myo10 (GFP-Myo10) and mutants were transiently expressed in HEK293 cells and were analyzed by immunoblotting with anti-GFP. The scheme shows the domain structures of GFP-Myo10. CC, coiled-coil domain; PEST, polypeptide enriched in proline, glutamic acid, serine, and threonine residues. (C and D) FLAG-ADD1 was transiently coexpressed with GFP-Myo10 or its mutants in HEK293 cells. GFP-Myo10 was immunoprecipitated (IP) by anti-GFP, and the immunocomplexes were analyzed by immunoblotting with anti-FLAG or anti-GFP. (E) GFP-Myo10-N was transiently coexpressed with (+) or without (−) the FLAG-ADD1Δtail mutant in HEK293 cells. The cell lysates were incubated with anti-FLAG, and the immunocomplexes were analyzed by immunoblotting with anti-GFP or anti-FLAG. (F) GFP-Myo10-N was transiently coexpressed with FLAG-ADD1 or its mutants in HEK293 cells. FLAG-ADD1 was immunoprecipitated by anti-FLAG, and the immunocomplexes were analyzed by immunoblotting with anti-GFP or anti-FLAG. (G) HeLa cells were infected with lentiviruses expressing shRNAs to Myo10 (sh-Myo10 #1 and sh-Myo10 #2) or to luciferase (sh-Luc.) as a control. The expression levels of Myo10, ADD1, and β-tubulin (as a loading control) were analyzed by immunoblotting with the indicated antibodies. (H) The cells, as in G, were stained for ADD1, α-tubulin, and DNA. Arrows indicate misaligned chromosomes. The percentage of ADD1 association with mitotic spindles in the total number of mitotic cells counted was measured (n > 270). Values (means ± SD) are from three independent experiments. **, P < 0.01. Bars, 5 µm.