Abstract
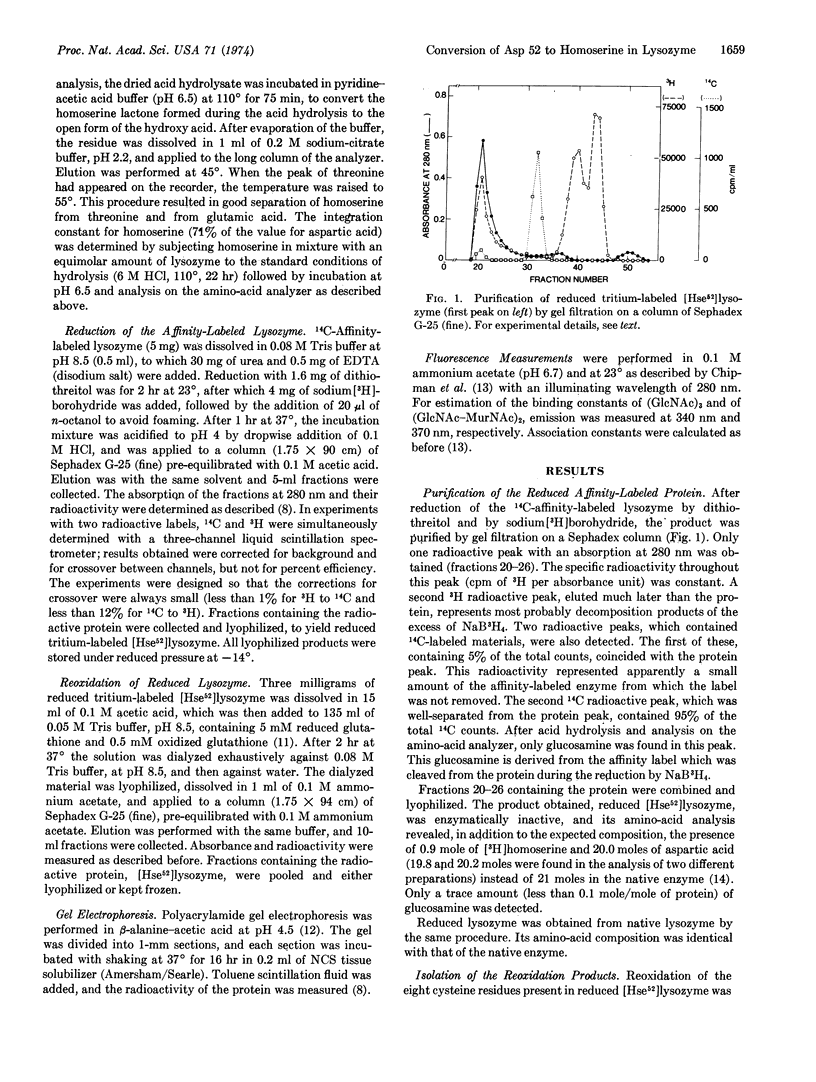
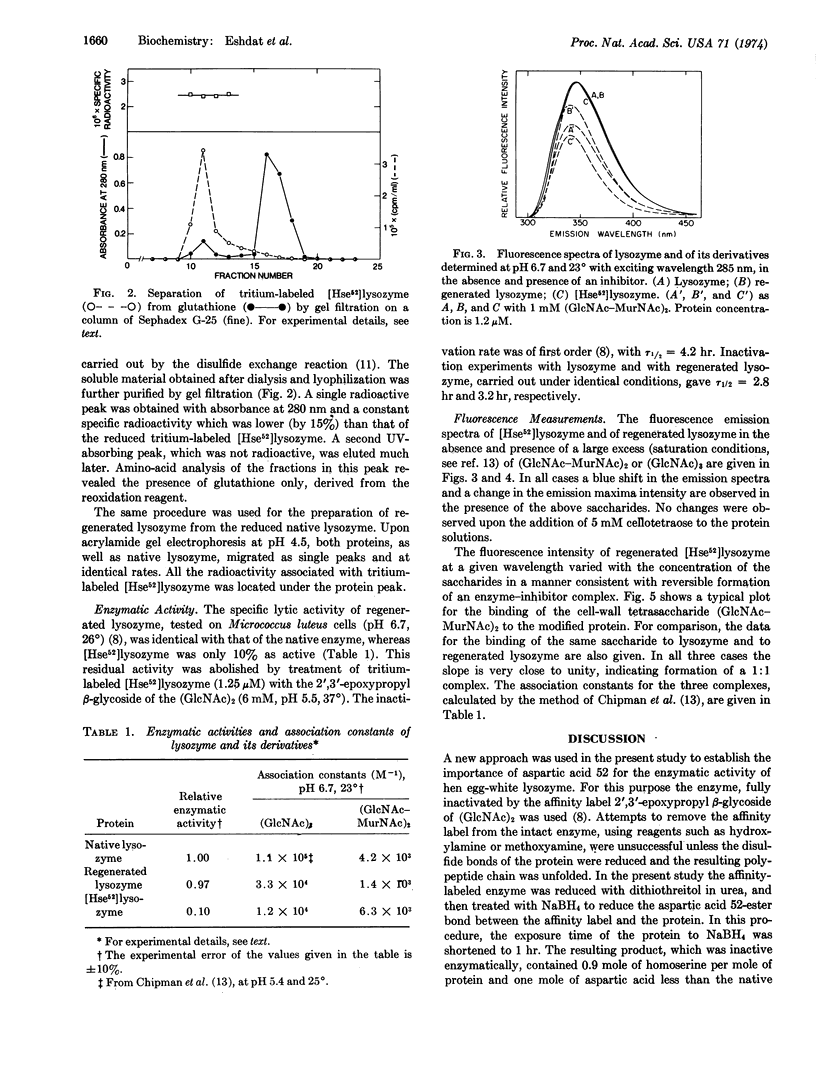
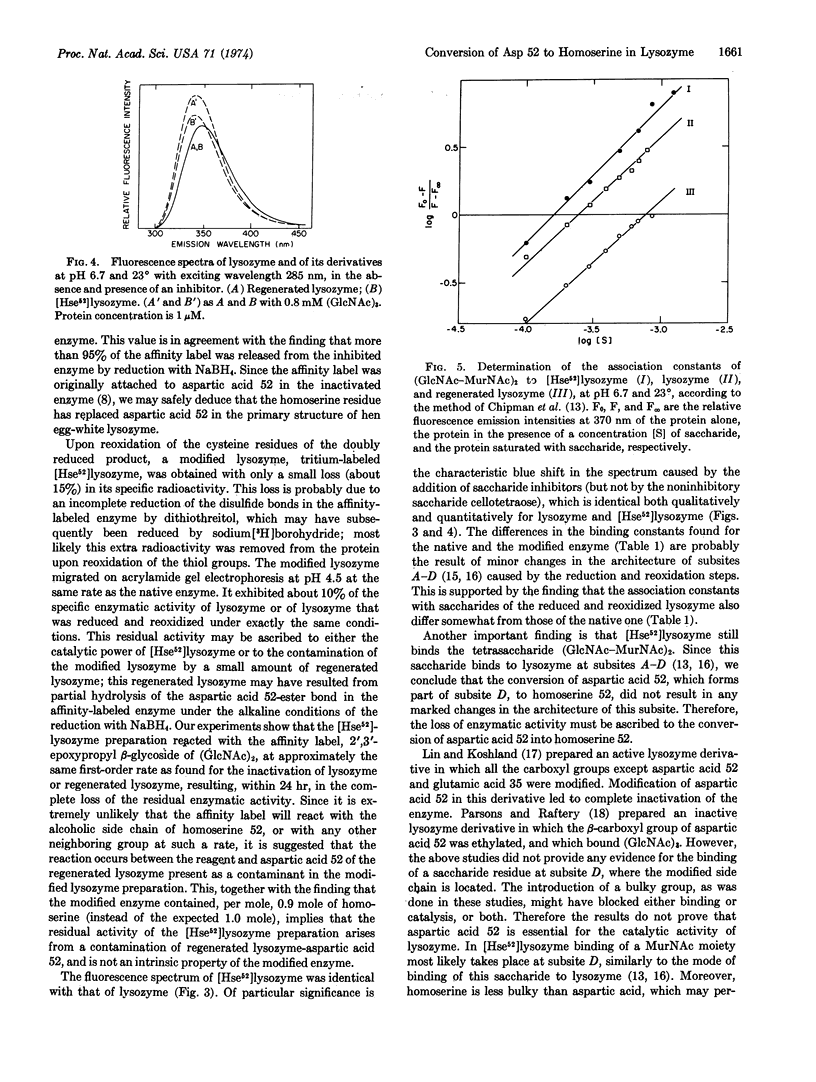
Hen egg-white lysozyme (EC 3.2.1.17) was specifically esterified at aspartic acid 52 by the affinity labeling reagent 2′,3′-epoxypropyl β-glycoside of di-(N-acetyl-D-glucosamine) [Eshdat et al. (1973) J. Biol. Chem.248, 5892]. The disulfide bonds of the affinity-labeled enzyme and the aspartic acid 52-ester bond were reduced with dithiothreitol and sodium borohydride, respectively, resulting in the removal of the affinity label. The reduced protein contained 0.9 mole of homoserine and 1 mole less of aspartic acid per mole of protein, as compared to the native enzyme. It was reoxidized by a mixture of reduced and oxidized glutathione to yield a modified protein that possessed one-tenth of the activity of native lysozyme (presumably due to a contamination by regenerated lysozyme formed as a result of hydrolysis of the aspartic acid 52-ester bond during the chemical treatment). The native enzyme, after reduction and reoxidation in the same manner, retained its amino-acid composition, full enzymatic activity, and fluorescence properties. The modified lysozyme, containing homoserine 52, showed the same fluorescence spectrum as the native enzyme. With both proteins, the fluorescence maximum shifted to the blue to a similar extent upon the addition of the saccharide inhibitors tri-(N-acetyl-D-glucosamine) and the cell-wall tetrasaccharide (GlcNAc-MurNAc)2. The modified enzyme bound these two saccharides with nearly the same binding constants as those found for native lysozyme and for lysozyme that was reduced and reoxidized. Since the side chain of homoserine is similar in size to that of aspartic acid, it is concluded that the loss of enzymatic activity is the direct result of the chemical modification of the carboxyl side chain of aspartic acid 52, thus showing that this amino acid is essential for the catalytic action of the enzyme.
Keywords: enzyme mechanism, specific chemical modification of proteins, affinity labeling, active site, ester bond reduction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANFIELD R. E. PEPTIDES DERIVED FROM TRYPTIC DIGESTION OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2691–2697. [PubMed] [Google Scholar]
- Chipman D. M., Grisaro V., Sharon N. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J Biol Chem. 1967 Oct 10;242(19):4388–4394. [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., McKelvy J. F., Sharon N. Identification of aspartic acid 52 as the point of attachment of an affinity label in hen egg white lysozyme. J Biol Chem. 1973 Aug 25;248(16):5892–5898. [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr The active site and enzyme action. Adv Enzymol Relat Subj Biochem. 1960;22:45–97. doi: 10.1002/9780470122679.ch2. [DOI] [PubMed] [Google Scholar]
- Lin T. Y., Koshland D. E., Jr Carboxyl group modification and the activity of lysozyme. J Biol Chem. 1969 Jan 25;244(2):505–508. [PubMed] [Google Scholar]
- Neet K. E., Koshland D. E., Jr The conversion of serine at the active site of subtilisin to cysteine: a "chemical mutation". Proc Natl Acad Sci U S A. 1966 Nov;56(5):1606–1611. doi: 10.1073/pnas.56.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. M., Raftery M. A. The identification of aspartic acid residue 52 as being critical to lysozyme activity. Biochemistry. 1969 Oct;8(10):4199–4205. doi: 10.1021/bi00838a043. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]


