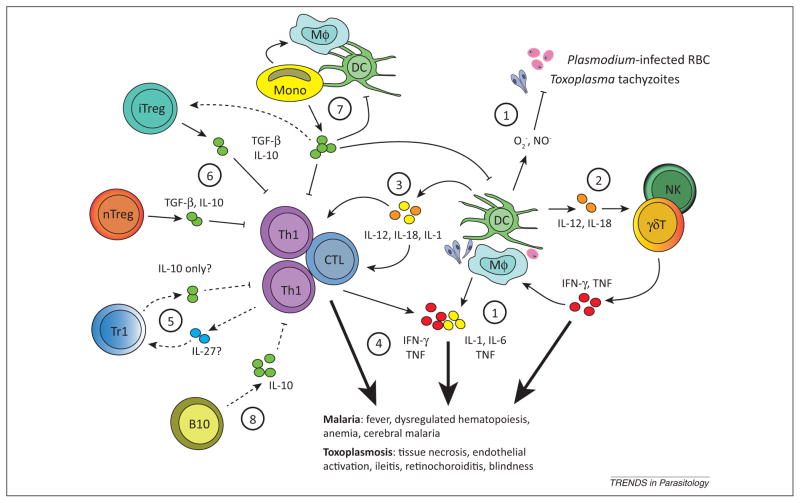
Figure 1.
Common regulatory networks limit Toxoplasma and Plasmodium immunopathogenesis. (1) Recognition of parasites or parasite-infected cells by macrophages (Mφ) and dendritic cells (DCs) triggers the production of antiparasitic reactive oxygen species and synthesis of inflammatory cytokines [88–92]. These inflammatory agents can act directly on parenchymal cells to induce necrotic or apoptotic pathways causing tissue damage, or (2) indirectly through the activation of innate natural killer (NK) or γδ T cells (γδT) [93,94]. (3) Cytokines such as IL-12, IL-1, and IL-18 also play a critical role in shaping the inflammatory nature of adaptive T cell subsets, which include IFN-γ secreting T helper cells (Th1) and cytotoxic cells (CTL) [85,93]. (4) Effector T cells express several inhibitory receptors including programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) that act to limit their activation, proliferation, and cytokine expression, which helps maintain immune homeostasis and limit immunopathology after infection [5,6,11,13]. Generally, and despite inhibitory receptor expression, during acute intestinal or ocular toxoplasmosis and malaria, highly activated T cells appear to directly contribute to pathology [37,46,98,104]. Pathological inflammatory reactions are themselves counterbalanced by several secreted regulatory cytokines and functionally distinct immune cell subsets. Regulatory cytokines such as IL-10 and TGF-β can be secreted by multiple cell types, including B cells, T cells, monocytes (Mono), Mφ, and DCs [2,17,18,24,26,29,51,67]. (5) Polarized IFN-γ-secreting Th1 cells also have the capacity to secrete IL-10 after their differentiation into Treg-like (Tr1) cells. Tr1 differentiation may involve IL-27 [23]. (6) Natural, thymus-derived Tregs (nTregs) suppress the activity of inflammatory CTL and Th1 cells through direct contact or secretion of IL-10 and TGF-β [12,46,106]. The precise role of nTreg, inducible Treg (iTreg), and Tr1 in limiting immunopathology during malaria and toxoplasmosis is not well understood. (7) Monocytes that infiltrate Toxoplasma-infected tissues or the spleen of Plasmodium-infected hosts exhibit dual roles in pathology and protection, by either secreting inflammatory cytokines (e.g., IL-12, TNF-α, IL-1, IL-6) or regulatory cytokines (IL-10 or TGF-β), and possibly by driving the differentiation of Tregs [52–56]. The cues that determine whether monocytes exhibit an inflammatory or regulatory function are unknown. Additional questions include the specific role of IL-27 and the cellular source(s) of this cytokine, as well as (8) the contribution of IL-10-secreting regulatory B cells (B10). Well-described pathways are shown as unbroken lines, and unknown or speculative pathways are shown as broken lines.