Abstract
Background. The effect of early initiation of antiretroviral therapy (ART; ie, at CD4+ T-cell counts >350 cells/mm3) on sexual behaviors and human immunodeficiency virus type 1 (HIV) transmission risk has not been documented in populations other than HIV-serodiscordant couples in stable relationships.
Methods. On the basis of data from a behavioral study nested in a randomized, controlled trial (Temprano-ANRS12136) of early ART, we compared proportions of risky sex (ie, unprotected sex with a partner of negative/unknown HIV status) reported 12 months after inclusion between participants randomly assigned to initiate ART immediately (hereafter, “early ART”) or according to ongoing World Health Organization criteria. Group-specific HIV transmission rates were estimated on the basis of sexual behaviors and viral load–specific per-act HIV transmission probabilities. The ratio of transmission rates was computed to estimate the protective effect of early ART.
Results. Among 957 participants (baseline median CD4+ T-cell count, 478 cells/mm3), 46.0% reported sexual activity in the past month; of these 46.0%, sexual activity for 41.5% involved noncohabiting partners. The proportion of subjects who engaged in risky sex was 10.0% in the early ART group, compared with 12.8% in the standard ART group (P = .17). After accounting for sexual behaviors and viral load, we estimated that the protective effect of early ART was 90% (95% confidence interval, 81%–95%).
Conclusion. Twelve months after inclusion, patients in the early and standard ART groups reported similar sexual behaviors. Early ART decreased the estimated risk of HIV transmission by 90%, suggesting a major prevention benefit among seronegative sex partners in stable or casual relationships with seropositive individuals.
Keywords: HIV prevention, antiretroviral treatment, sexual behaviors, HIV-1 sexual transmission, treatment as prevention, epidemiology, sub-Saharan Africa
By controlling viral replication, antiretroviral therapy (ART) reduces the infectivity of human immunodeficiency virus type 1 (HIV)–positive patients, with some evidence that patients with an undetectable viral load have a negligible HIV transmission risk [1, 2]. In 2011, the HPTN052 trial demonstrated that initiating ART at a CD4+ T-cell count of 350–550 cells/mm3, which is greater than the CD4+ T-cell count of <350 cells/mm3 recommended in 2010 by World Health Organization (WHO) guidelines, had a 96% preventive effect against HIV transmission among HIV-serodiscordant couples in a stable relationship [3]. This evidence strengthened the treatment-as-prevention concept, under which providing ART to all HIV-infected patients, regardless of their CD4+ T-cell count, might decrease HIV transmission among the general population to such an extent that it would curtail the HIV pandemic [4, 5]. Results of randomized, controlled trials of treatment as prevention are not expected for several years [6]. Meanwhile, there are still questions regarding factors that might impact the effect of early ART in the general population and make this effect different from that observed in the very specific group of stable serodiscordant couples.
Among these factors, the influence of early ART on risky sexual behaviors is one of major concern. In the first years of the highly active ART era, increased risky sexual behaviors associated with ART were reported among high-risk groups [7, 8]. Risk compensation related to decrease in perception of HIV transmission risk and severity of HIV infection may potentially offset the protective effect of early ART [9, 10]. Nevertheless, a recent review of observational studies conducted in developing countries instead suggested a decrease in risky sexual behaviors after ART initiation [11]. However, such studies may be subject to confounding. In addition, only a few were prospective [12], and all were conducted in the context of standard ART initiation [11, 13]. Starting ART earlier, while patients are healthy, may influence sexual behaviors differently. Updating results about this issue, especially in the current context of early ART initiation, is thus needed [14].
Evidence obtained so far has resulted in WHO recommendations of early ART for prevention specifically for the population of serodiscordant couples [15]. Nevertheless, according to a recent estimation, only less than a third of new HIV transmissions in sub-Saharan Africa occur among these couples [16]. This suggests that programs solely targeting stable serodiscordant couples may lack the ability to prevent the majority of new infections. Estimating the preventive effect of early ART beyond the population of stable serodiscordant couples is thus of great interest for scaling up effective prevention strategies.
Relying on data from the ongoing Temprano-ANRS12136 randomized, controlled trial, we aimed to measure the impact of early ART initiation on sexual behaviors and to estimate its protective effect among a West African adult population reporting diverse heterosexual partnership statuses (ie, serodiscordant, seroconcordant, stable, or casual).
MATERIAL AND METHODS
Temprano-ANRS12136 Trial
Temprano is a multicenter, randomized, open-label superiority trial to assess the benefits and risks of initiating ART earlier than currently recommended by the WHO, concomitantly or not with 6-month isoniazid prophylaxis for tuberculosis. The trial was launched in March 2008 in Abidjan, Côte d'Ivoire, and is still ongoing. The trial protocol was approved by the ethics committee of the Ministry of Health of Côte d'Ivoire and by the institutional review board of the French National Agency for Research on AIDS and Viral Hepatitis (ANRS; Paris, France). The study has been registered on Clinicaltrials.gov (clinical trials identifier NCT00495651).
Between March 2008 and July 2012, patients attending 9 care centers in Abidjan were included in the trial whenever they provided signed, informed consent; were aged >18 years; had HIV-1 infection or HIV-1/HIV-2 dual infection; had no ongoing active tuberculosis; had no ongoing pregnancy and/or were not breast-feeding; and had a CD4+ T-cell count <800 cells/mm3 and satisfied no criteria for starting ART, according to the most recent WHO guidelines. Participants were randomly assigned to one of 4 arms: 2 “standard ART” arms (arms 1 and 2), in which ART was delayed until patients meet ongoing WHO starting criteria [17, 18]; and 2 “early ART” arms (arms 3 and 4), in which ART was initiated immediately on inclusion. In arm 2 and arm 4, participants received 6-month isoniazid prophylaxis for tuberculosis, starting at the 1-month visit and stopping at the 7-month visit. Once included, participants were asked to show up for trial scheduled visits every 3 months. CD4+ T-cell count and plasma HIV-1 RNA load (real-time polymerase chain reaction, TaqMan technology; ABI Prism 7000, Applied Biosystems; lower limit of detection, 300 copies/mL) were measured every 6 months. Each participant was to be followed during 30 months. The main outcome of the trial is the occurrence of a new episode of severe morbidity and any event leading to death.
Sociobehavioral Study
The present sociobehavioral study was nested in the Temprano trial. All participants included in Temprano between 1 January 2009 and 1 September 2011 were eligible for the study. A standardized questionnaire was used to collect information on sexual behaviors. Participants completed this questionnaire face to face at the 12-month visit.
Study Outcomes
Sexual behaviors of interest included sexual activity and multiple partnerships in the past year, as well as characteristics of the last episode of sexual intercourse, including date (occurrence in the past month or the past year), type of partnership (cohabiting or not), and partner's HIV status (unknown, negative, or positive).
Risky sex at the last episode of sexual intercourse in the past month was defined as an episode of unprotected intercourse with a partner whose HIV status was negative or unknown. Partner's exposure to HIV infection was defined as risky sex at the last episode of sexual intercourse in the past month plus a viral load of >300 copies at the time of the intercourse, i.e. measured within a period ranging from 30 days before to 7 days after the date of completion of the sociobehavioral questionnaire.
For each sexually active participant, the risk of HIV transmission during the last episode of intercourse in the past month was calculated on the basis of the reported partner's HIV status, condom use, and viral load measured at the time of the intercourse. Per-coital-act viral load–specific probabilities of transmission were derived from a seroconversion study of HIV-discordant couples in eastern and southern Africa [19], using the following formula:
where p is the per-coital probability of HIV transmission (J. P. Hughes, personal communication).
Patients with an undetectable viral load were assigned a null transmission risk [2]. We attributed a 78% transmission risk reduction if condom use was reported, and for female participants we considered each last male sex partner as circumcised (96% of men actually are in Côte d'Ivoire [20]) and thus used a 53% transmission risk reduction [19]. Partners with an unknown HIV status were considered as HIV uninfected. For a partner reported as HIV positive, the transmission risk was set to 0.
We estimated HIV transmission rates at the last episode of sexual intercourse in the past month for both ART strategies by computing mean values of individual transmission risks, expressed as the number of transmissions per 10 000 sexually active individuals. To estimate transmission rates overall rather than among sexually active persons only, we computed an additional estimate that included participants who were sexually inactive in the past month, attributing to them a null individual transmission risk.
Statistical Analysis
Participants included in the Temprano trial between 1 January 2009 and 1 September 2011 were included in this analysis provided they completed the sociobehavioral questionnaire within 9–15 months after enrollment in the Temprano trial. Analyses were conducted on the basis of intention to treat. Sexual behaviors of interest were compared between early ART and standard ART strategies, using χ2 tests. The protective effect of the early ART strategy on the HIV transmission risk was based on the ratio of transmission rates.
To assess the robustness of our estimates, we conducted a range of sensitivity analyses, considering (1) only participants with a baseline CD4+ T-cell count of > 350 cells/mm3, (2) only those engaged in a cohabiting serodiscordant relationship, (3) a nonnull transmission probability for those having a viral load of < 300 copies/mm3 [21], (4) an alternative data set for viral load–specific transmissions probabilities [22], (5) an HIV-positivity probability of 0.4 for a sex partner with an unknown serostatus, (6) all participants as having had an HIV-negative partner during the last episode of unprotected sexual intercourse.
All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC). We computed 95% confidence intervals (CIs) for expected transmission rates and the protective effect of early ART, using bootstrapping (10 000 resampling).
RESULTS
Study Population
Among the 1172 participants included in the Temprano trial between 1 January 2009 and 1 September 2011, 957 (81.7%) were included in the analyses (standard ART group, 467 patients; early ART group, 490 patients). Participants in the early and standard ART groups attended a similar mean number of trial medical visits between enrollment and 12 months (standard ART group, 5.9 visits; early ART group, 6.2 visits).
The remaining 215 patients (123 in the standard ART group vs 92 in the early ART group; P = .03) were excluded for the following reasons: death within the first 12 months (6 and 9, respectively; P = .42), not showing up for the 12-month visit (17 and 38, respectively; P = .003); 12-month sociobehavioral questionnaire not or untimely completed (93 [of whom 59 had initiated ART before the 12-month visit] and 30, respectively; P = .02).
Participants included in the study did not differ from those who were excluded with regard to sociodemographic characteristics, except for education level (47.1% of patients included, compared with 38.1% of those excluded, had at least a secondary level of education; P = .02).
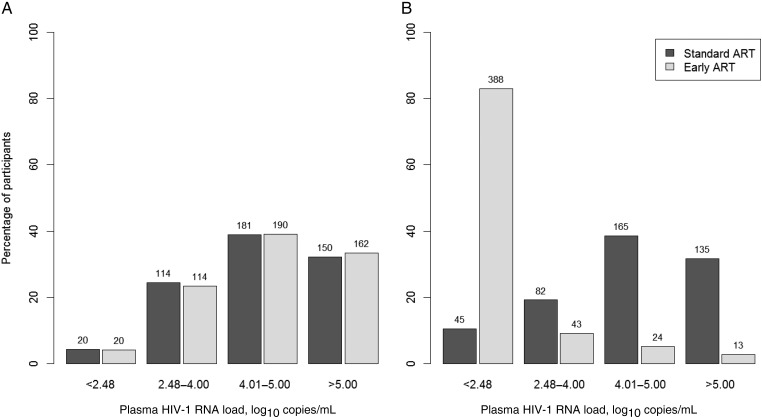
Among the 957 participants, a large majority (80.4%) were women. At baseline, the median age was 35 years, and 442 participants (46.2%) were living in union. The median CD4+ T-cell count was 478 cells/mm3. Neither baseline sociodemographic and clinical characteristics (Table 1) nor baseline viral load distributions (P=.53, by the Wilcoxon rank sum test; Figure 1A) significantly differed between participants in the early and standard ART groups.
Table 1.
Baseline Characteristics, by Antiretroviral Therapy (ART) Strategy
| Characteristic | Standard ART, Patients, No. (%) (n = 467) | Early ART, Patients, No. (%) (n = 490) | P |
|---|---|---|---|
| Sex | .78 | ||
| Male | 93 (19.9) | 94 (19.2) | |
| Female | 374 (80.1) | 396 (80.8) | |
| Age, y | .30 | ||
| <30 | 118 (25.3) | 118 (24.1) | |
| 30–40 | 217 (46.4) | 211 (43.1) | |
| >40 | 132 (28.3) | 161 (32.8) | |
| Education level | .10 | ||
| None | 94 (20.1) | 131 (26.7) | |
| Primary | 144 (30.9) | 137 (28.0) | |
| Secondary | 170 (36.4) | 170 (34.7) | |
| >Secondary | 59 (12.6) | 52 (10.6) | |
| Personal source of income | .44 | ||
| No | 116 (26.1) | 134 (28.3) | |
| Yes | 329 (73.9) | 339 (71.7) | |
| Family status | .52 | ||
| Single | 200 (42.8) | 203 (41.4) | |
| Living in union | 218 (46.7) | 224 (45.7) | |
| Separated/widowed | 49 (10.5) | 63 (12.9) | |
| Perceived health | .12 | ||
| Excellent/very good | 100 (21.7) | 99 (20.5) | |
| Good | 298 (64.6) | 295 (60.9) | |
| Poor/bad | 63 (13.7) | 90 (18.6) | |
| WHO clinical stage | .97 | ||
| 1 | 290 (62.1) | 310 (63.3) | |
| 2 | 125 (26.8) | 124 (25.3) | |
| 3 | 50 (10.7) | 54 (11.0) | |
| 4 | 2 (0.4) | 2 (0.4) | |
| CD4+ T-cell count, cells/mm3 | .44 | ||
| <350 | 71 (15.2) | 88 (17.9) | |
| 350–499 | 176 (37.7) | 187 (38.2) | |
| ≥500 | 220 (47.1) | 215 (43.9) |
Data are from 957 patients who participated in a sociobehavioral study nested in the Temprano trial and were recorded at the 12-month visit. Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met.
Abbreviation: WHO, World Health Organization.
Figure 1.
Distribution of plasma human immunodeficiency virus type 1 (HIV-1) RNA, by antiretroviral (ART) strategy at baseline (A) and 12 months after inclusion (B). A, In the standard ART group, 4.3% had an undetectable viral load at baseline, and the mean viral load among individuals with a detectable viral load was 4.60 log10 copies/mL (95% confidence interval [CI], 4.52–4.68). In the early ART group, 4.1% had an undetectable viral load at baseline, and the mean viral load among individuals with a detectable viral load was 4.63 log10 copies/mL (95% CI, 4.55–4.71). B, In the standard ART group, 12.5% had an undetectable viral load 12 months after inclusion, and the mean viral load among individuals with a detectable viral load was 4.68 log10 copies/mL (95% CI, 4.60–4.76). In the early ART group, 82.9% had an undetectable viral load 12 months after inclusion, and the mean viral load among individuals with a detectable viral load was 3.88 log10 copies/mL (95% CI, 3.66–4.11). Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met. A log10 HIV-1 RNA load of <2.48 log10 copies/mL corresponds to an HIV-1 RNA detectability threshold of <300 copies/mL.
At the 12-month visit, 70 patients (15.0%) in the standard ART group had initiated ART (median duration between inclusion and ART initiation, 9.5 months). Overall, viral load measured at 12-month was available for 427 patients (91.4%) receiving standard ART and 468 (95.5%) receiving early ART. As expected, because of the difference in ART coverage, the percentage of patients with an undetectable viral load was significantly lower among those receiving standard ART, compared with those receiving early ART (P < 10−3, by the Wilcoxon rank sum test; Figure 1B).
Sexual Behaviors, Risky Sex, and Partner's Exposure to HIV at the 12-Month Visit
Sexual behaviors in the past 12 months are presented in Table 2. No significant difference was observed between patients receiving standard versus early ART in the proportions of sexual activity (71.7% in the standard ART group vs 69.8% in the early ART group; P = .51) and multiple partnerships (6.2% vs 9.0%, respectively; P = .11) in the past year. Among sexually active participants, 41.2% in the standard ART group and 41.8% in the early ART group reported they were not cohabiting with their last sex partner (P = .87). Overall, the last sex partner was reported to be HIV uninfected by 26.6% in the standard ART group, compared with 22.8% in the early ART group, and to have an unknown HIV-status by 43.9% and 47.7%, respectively (P = .47).
Table 2.
Sexual Behaviors in the Past 12 Months, by Antiretroviral Therapy (ART) Group
| Characteristic | Standard ART, Patients, Proportion (%) | Early ART, Patients, Proportion (%) | P |
|---|---|---|---|
| All patients | |||
| Sexually active in the past year | 335/467 (71.7) | 342/490 (69.8) | .51 |
| Multiple partnerships | 29/467 (6.2) | 44/490 (9.0) | .11 |
| Patients sexually active in the past year | |||
| Last intercourse with a cohabiting partner | |||
| Yes | 197/335 (58.8) | 199/342 (58.2) | .87 |
| No | 138/335 (41.2) | 143/342 (41.8) | |
| Last partner's HIV status | |||
| HIV negative | 89/335 (26.6) | 78/342 (22.8) | .47 |
| HIV positive | 99/335 (29.6) | 101/342 (29.5) | |
| Unknown | 147/335 (43.9) | 163/342 (47.7) | |
Data are from 957 patients who participated in a sociobehavioral study nested in the Temprano trial and were recorded at the 12-month visit. Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met.
Abbreviation: HIV, human immunodeficiency virus type 1.
Characteristics of the last episode of intercourse in the past month are presented in Table 3. Overall, 46.0% reported sexual activity in the past month; of these 46.0%, sexual activity for 41.5% involved noncohabiting partners. Participants receiving standard versus early ART did not significantly differ as regard to sexual activity in the past month and condom use. Risky sex was reported by 12.8% of participants in the standard ART group, compared with 10.0% in the early ART group (P = .54). When taking into account the last available viral load, the proportions of participants exposing their partner to HIV infection were 10.7% in the standard ART group, compared with 2.4%, in the early ART group (P < .001).
Table 3.
Characteristics of the Last Episode of Intercourse in the Past Month, by Antiretroviral Therapy (ART) Group
| Characteristic | Standard ART, Patients, No. (%) (n = 467) | Early ART, Patients, No. (%) (n = 490) | P |
|---|---|---|---|
| Last intercourse in the past month | 226 (48.4) | 214 (43.7) | .14 |
| Unprotected sex at last intercoursea | 100 (21.4) | 76 (15.5) | .06 |
| Risky sexb at last intercoursea | 60 (12.9) | 49 (10.0) | .54 |
| Partner's exposure to HIVc at last intercoursea | 50 (10.7) | 12 (2.45) | <10−3 |
Data are from 957 patients who participated in a sociobehavioral study nested in the Temprano trial and were recorded at the 12-month visit. Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met.
Abbreviation: HIV, human immunodeficiency virus type 1.
a Last intercourse in the past month.
b Unprotected sex with a partner of HIV-negative/unknown status.
c Unprotected sex with a partner of HIV-negative/unknown status and an HIV load of >300 copies/mL.
Estimated HIV-Transmission Rates at the 12-Month Visit
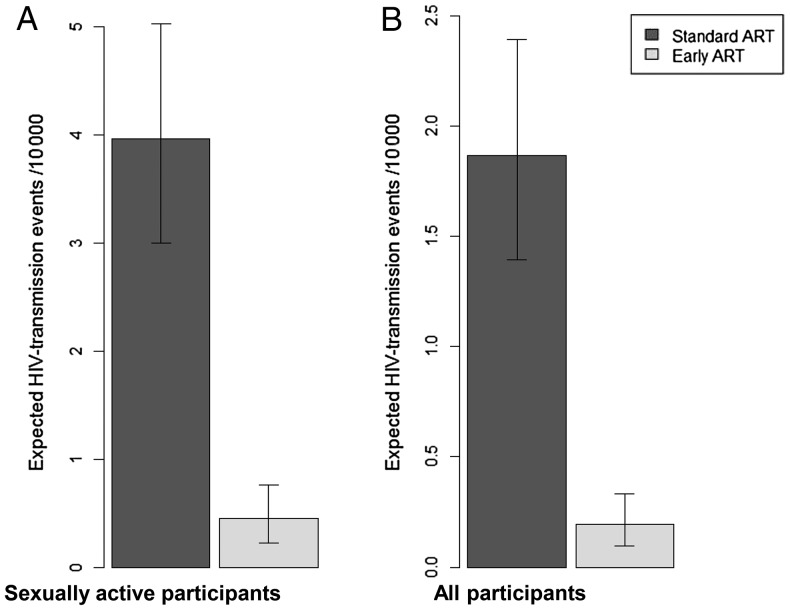
Figure 2A shows the estimated HIV transmission rates per 10 000 sexually active persons at the last episode of sexual intercourse in the past month, based on risk behaviors and viral load data, for both ART strategies. The estimated transmission rate was 4.0 cases/10 000 (95% CI, 3.0–5.0) among those receiving standard ART and 0.5 cases/10 000 (95% CI, .2–.8) among those receiving early ART. The corresponding estimated protective effect of early ART against HIV transmission was 89% (95% CI, 79%–95%). When including all participants in the computations (Figure 2B), the estimated transmission rate was 1.9 cases/10 000 (95% CI, 1.4–2.4) among those receiving standard ART and 0.2 cases/10 000 (95% CI, .1–.3) among those receiving early ART, representing a protective effect of 90% (95% CI, 81%–95%).
Figure 2.
Estimated human immunodeficiency virus type 1 (HIV)–transmission rates at the last episode of sexual intercourse in the past month, by antiretroviral therapy (ART) strategy, per 10 000 sexually active participants (A; preventive effect, 89% [95% confidence interval {CI}, 79–95]) and per 10 000 participants (B; preventive effect, 90% [95% CI, 81–95]). Data are from 957 patients who participated in a sociobehavioral study nested in the Temprano trial and were recorded at the 12-month visit. Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met. Per-act viral load–specific transmission probabilities are derived from Hughes et al [19]. Calculations accounted for sexual activity, condom use, circumcision, and partner's HIV infection status.
Results of sensitivity analyses are presented in Table 4. Whereas estimates of transmission rates varied substantially across scenarios, the estimated protective effect of early ART remained robust (ranging from 84% to 90%).
Table 4.
Sensitivity Analyses of Estimated Human Immunodeficiency Virus type 1 (HIV)–Transmission Rates at the Last Episode of Intercourse in the Past Month, by Study Arm, and Estimated Protective Effect of the Early Antiretroviral Therapy Strategy
| Population | Participants by Arm, No. | Specific Assumption | Expected HIV Transmissions by Arm, No./10 000 Persons (95% CI)a |
Protective Effect, % (95% CI) | |
|---|---|---|---|---|---|
| Standard | Early | ||||
| Total sample | Standard, 467; early, 490 | Main analysis | 1.87 (1.39–2.39) | 0.20 (.09–.33) | 90 (81–95) |
| Baseline CD4+ T-cell count >350 cells/mm3 | Standard, 396; early, 402 | Same as in the main analysis | 2.03 (1.47–2.66) | 0.20 (.08–.36) | 90 (81–96) |
| Cohabiting serodiscordant couples | Standard, 55; early, 54 | Same as in the main analysis | 3.36 (2.11–4.78) | 0.37 (.14–.64) | 89 (77–96) |
| Total sample | Standard, 467; early, 490 | viral load = 300 copies/mL for patients with undetectable viral load | 1.88 (1.40–2.40) | 0.30 (.19–.43) | 84 (75–90) |
| Total sample | Standard, 467; early, 490 | Same as in the main analysis | 1.72 (1.36–2.11)b | 0.25 (.14–.4)b | 85 (75–92) |
| Total sample | Standard, 467; early, 490 | 40% of partners with unknown HIV status considered as HIV-positive | 1.34 (1.01–1.70) | 0.13 (.07–.22) | 90 (82–95) |
| Total sample | Standard, 467; early, 490 | All participants considered as having had a last unprotected intercourse with a HIV-negative partner | 18.4 (16.6–20.3) | 2.1 (1.4–2.9) | 89 (84–92) |
Data are from 957 patients who participated in a sociobehavioral study nested in the Temprano trial and were recorded at the 12-month visit. Patients in the early arm initiated ART immediately on inclusion in the trial, whereas patients in the standard arm delayed ART initiation until ongoing WHO starting criteria were met.
a Data are expected no. of transmissions at the last episode of sexual intercourse in the past month. All data are from Hughes et al [19], unless otherwise indicated.
b Data for viral load–specific HIV transmission probabilities are from Gray et al [22].
Relying on the estimated distribution of coital frequency previously reported among serodiscordant couples [22], we assumed a monthly number of 8 episodes of sexual intercourse for those sexually active in the past month (0 episodes were assumed for those inactive) and extrapolated the characteristics reported for the last intercourse to all episodes of intercourse. Based on this assumption and on our estimate of transmission rates at last sexual intercourse calculated in the whole studied population, we estimated that early ART, compared with a standard ART strategy, could prevent 13.4 infections/10 000 patients (95% CI, 9.4–17.7) during the 12-month period following early ART initiation. By extrapolating this monthly number to the whole period (months 0–12), we estimated that early ART could prevent 161 infections/10 000 patients (95% CI, 113–212) in their first year of treatment (based on estimated incidence rate of 18.7 infections/10 000 persons-years in the early ART group and 179.0 infections/10 000 person-years in the standard ART group).
DISCUSSION
In this study, which was nested in an ongoing randomized trial of early ART, patients who initiated ART at high CD4+ T-cell counts and those who delayed ART initiation until WHO criteria were met declared similar sexual behaviors at 12 months. Early ART was estimated to decrease the risk of HIV transmission to partners by 90%. This estimated risk reduction, which accounted for well-identified determinants of HIV transmission, including viral load, sexual partnership, condom use, and circumcision [19], was mainly attributable to differences in viral loads between patients receiving early versus standard ART. In contrast with previous studies that demonstrated the protective effect of ART among serodiscordant stable couples, these estimates were derived from a diverse population with a wide range of partnerships and HIV statuses of partners. More than half of our patients were not in a cohabiting relationship, and about two-thirds of those who were sexually active reported that their last partner was HIV negative or had an unknown HIV status.
The first very original finding of this study involves sexual behaviors among patients receiving early ART, which were comparable to those reported by patients receiving standard ART 12 months after inclusion. The sample size was large enough to allow the detection of a 5% difference (from 5% to 10%) between both ART strategies, with a power of 0.95. Overall proportions of sexual activity in the past year (71%) and no use of protection during the last episode of intercourse (25.4%) were consistent with data previously reported in Côte d'Ivoire for patients treated at late stages of HIV infection [23–25]. To our knowledge, no data on sexual behaviors in the context of early ART have been published to date.
According to a recent review conducted in developing countries, a decrease in unprotected sex was observed in 16 of 17 observational studies among patients on standard initiated treatment [11]. Such a decline in unprotected sex associated with ART may be explained by the multiple medical encounters that treated patients have with the care system, which ensures a high level of prevention counseling and psychosocial support [25, 26]. Routine contact with the healthcare system is often rare for patients who are not eligible for ART [27]. In our study, participants in the early and standard ART groups had a high frequency of contact with medical care, which may explain comparability in self-reported sexual behaviors. Because data reported in the standard ART initiation context show that sexual behavior changes may occur over a long period [28], sexual behavior changes in relation to early ART initiation deserve to be further assessed over the long term.
We used 3 different indicators to estimate HIV transmission risk and its reduction. The first indicator was the proportion of last episodes of sexual intercourse exposing the partner to HIV infection, based on the plasma viral load. This proportion was significantly lower among participants receiving early versus standard ART. Proportions of unprotected episodes of intercourse with serodiscordant partners regardless of viral load were not significantly different between both ART strategies. This suggests that the protective effect of early ART versus a standard strategy principally lies on the biological effect of the treatment on viral replication rather than on a combination of biological and behavioral effect, as suggested by others [11].
Our second estimate of HIV transmission risk was the expected number of transmissions at the last episode of intercourse in the past month, combining sexual behavior (condom use, partner's HIV status, and circumcision), exact viral loads, and HIV-1 transmission probabilities from the literature [19]. This method has been recently used and showed consistent results as compared to a seroconversion study [29]. On the basis of this method, we estimated a protective effect of 90% for early ART. The magnitude of the protective effect we found was remarkably consistent across populations considered in the analysis, changes in our assumptions, and variations in the parameters (Table 4), arguing for the robustness of our estimate. Moreover, this protective effect was close to those estimated in the HPTN052 study (96%) [3] and in a systematic review of prospective studies among discordant couples (91%; 95% CI, 79%–96%) [30].
Third, we assessed the protective effect of early ART by computing the number of infections averted yearly for 10 000 persons after 1 year of treatment, which we estimated between 113 and 212. Those results rely on strong assumptions regarding the frequency of sexual intercourse and the stability over time of the ART preventive effect in the first year of treatment. Despite the uncertainty surrounding these assumptions, our results are quite consistent with those from previous studies. The sensitivity analysis we conducted on the subsample of stable serodiscordant couples allowed us to estimate HIV incidence rates, which were in the same range than those reported by the HPTN052 study [3]. Moreover, a previous model estimated that providing early ART (at CD4+ T-cell counts of 350–500 cells/mm3) to serodiscordant couples might be expected to avert 210 infections per 10 000 person-years of ART [31]. Our estimate of 161 infections averted/10 000 is lower, which is consistent with the fact that our study included a broader population than serodiscordant couples, a substantial portion of whom (ie, those who are sexually inactive and/or engaged in seroconcordant partnerships) do not benefit from the preventive effect of ART.
Our results were obtained in a population with an early HIV diagnosis who were engaged in a 30-month trial. The sex ratio was unbalanced in favor of women, which reflects both the sex-specific prevalence of HIV infection (6.4% and 2.9%, respectively [20]) and delayed diagnosis among men, who have lower opportunities for early diagnoses than women in Côte d'Ivoire [32]. Even if participants of the present study potentially constitute a compliant population engaged in a trial offering good care conditions, the proportion of viral suppression achievement in participants receiving early ART 12 months after enrollment (83%) was not dramatically higher than that documented in population-based studies throughout sub-Saharan Africa [33]. This suggests that our results are likely to be in the range of figures observed more widely in West Africa.
Our study has some limitations. First, the present behavioral study was nested in a randomized, controlled clinical trial whose primary objective was to measure individual rather than collective benefits and risks of early ART. Thus, before the implementation of the 2012 WHO guidelines [15], our results were obtained in the absence of specific information about the preventive effect of ART. The current study may therefore only partially address the issue of risk compensation. Further research questioning risk perception in the context of widely available information about the preventive effect of early ART is needed, both among HIV-infected and HIV-uninfected people.
Second, the results of this study largely rely on self-reported sexual behaviors, which are potentially subject to social desirability bias. Overreport of condom use could lead to an underestimation of the estimated HIV transmission risks. However, since counseling and follow-up were similar between both ART groups, such a bias is unlikely to be differential and, thus, to have affected our estimate of the preventive effect of early ART.
Third, estimates of HIV transmission risk were based on the characteristics of the last episode of sexual intercourse. This might have biased the analysis if the frequency of sexual intercourse differed between both groups and/or if there were differences between groups in the extent to which the last episode of sexual intercourse reflected overall sexual behaviors. Such differences are unlikely, though, given that both groups were comparable for various indicators, including sexual activity and multiple partnerships.
Fourth, differences between ART strategies in the proportion of nonresponses to the month 12 questionnaire might have biased estimations of HIV transmission risk. A lower proportion of participants receiving standard versus early ART completed the questionnaire during the considered window period (ie, between months 9 and 15). Patients who completed the questionnaire outside of this period were mostly individuals randomly assigned to the standard ART group who initiated ART during the first 12 months of follow-up, which rescheduled subsequent visits and questionnaire completions from the date of ART initiation. Compared with other patients receiving standard ART, patients who started treatment before the 12-month visit probably had a higher viral load and, therefore, higher infectiousness during their pre-ART period, followed by a lower viral load and infectiousness after initiating ART. When considering the whole period (ie, from months 0–12), excluding these patients may have led to limited bias in the estimates of the transmission risk among patients receiving standard ART and of the protective effect of early ART. In addition, missing the 12-month visit during the window period was more frequent among patients in the early ART group (38 vs 17). In 2011, because of the political crisis faced by Côte d'Ivoire, the Temprano staff anticipated violence in Abidjan and predictable disruption of health services by giving in advance a higher stock of drugs to patients [34]. Thus, treated patients might have delayed their 12-month visit without being necessarily out of treatment.
Expanding ART coverage has resulted in a decreased HIV infection incidence in South Africa [35], but other natural experiments showed limited effect on HIV transmission, especially when risk compensation was observed [36]. Community trials have started to formally assess the effect of early ART on HIV infection incidence, but their results will not be released for several years. Meanwhile, our results suggest a strong protective effect of early ART on HIV heterosexual transmission, without any detectable effect on sexual behaviors. This effect was estimated in a population that included substantial proportions of persons out of stable partnership or with a seroconcordant partner. This population was thus more similar to the whole HIV-infected population than in previous studies, restricted to stable serodiscordant couples. The WHO has recently recommended early ART initiation for serodiscordant couples [15]. The social acceptability and equity of prioritizing access to early ART to this population is questionable, though [37]. Recent modeling studies on the contribution of HIV transmissions occurring among stable serodiscordant couples to the global sub-Saharan HIV epidemics demonstrated that prevention interventions targeted solely those couples may have a limited public health impact [16, 38]. Our results provide evidence for the public health significance of early ART initiation among a wider segment of the HIV-infected population.
ANRS 12136 TEMPRANO TRIAL GROUP
Clinical care in Abidjan, Côte d'Ivoire: Emmanuel Bissagnene, Serge Eholie (principal investigator), Gustave Nzunetu, Cyprien Rabe, and Sidibé Baba (Service des Maladies Infectieuses et Tropicales); Olivier Ba-Gomis, Henri Chenal, Marcelle Daligou, and Denise Hawerlander (Centre Intégré de Recherches Biocliniques d'Abidjan); Lambert Dohoun, Seidou Konate, Albert Minga, and Abo Yao (Centre National de Transfusion Sanguine); Constance Kanga, Koulé Serge, Jonas Séri, Calixte Guéhi, and Fassiri Dembélé (Unité de Soins Ambulatoires et de Conseil); Eugène Messou, Amani Anzian, Joachim Gnokoro, and Patrice Gouessé (Centre de Prise en Charge et de Formation); Madeleine Kadio-Morokro, Alain Kouadio, Séna Gountodji, Ediga Yédjédji, and Alexis Amian (La pierre angulaire); Emmanuel Kouamé, Dominique Koua, Solange Amon, Laurent Dja-Beugré, and Amadou Kouamé (Hôpital Général Abobo Nord); Oyéounlé Makaïla, Mounkaila Oyébi, Stanislas Sodenougbo, and Nathalie Mbakop (FSU Anonkoua kouté); and Babatundé Natanael, Babatundé Carolle, Gisèle Bléoué, and Mireille Tchoutchedjem (Centre de santé El Rapha). Biology: Matthieu Kabran (bacteriologist), Arlette Emieme (monitor), André Inwoley (immunologist), Hervé Menan (parasitologist), Timothée Ouassa (bacteriologist), Thomas-d'Aquin Toni (virologist), and Vincent Yapo (virologist; Centre de Diagnostic et de Recherches sur le SIDA, CHU de Treichville, Abidjan, Côte d'Ivoire); and Marie-Laure Chaix (virologist) and Christine Rouzioux (virologist; Service de Virologie, CHU Necker, Paris, France). Trial coordination team: Xavier Anglaret (principal investigator), Christine Danel (coordinator), Raoul Moh (coordinator), Romuald Konan (pharmacist), Anani Badjé (monitor), Jean Baptiste N'takpé (monitor), Gérard Menan Kouamé (monitor), and Franck Bohoussou (data manager; Programme PACCI, Abidjan, Côte d'Ivoire); and Delphine Gabillard (statistician) and Jérôme Le Carrou (monitor; Centre Inserm 897, Bordeaux, France). Trial steering committee: Jean-Marie Massumbuko, Emmanuel Bissagnene, Géneviève Chêne, Kouao Domoua, Mireille Dosso, Pierre-Marie Girard, Vincent Jarlier, Christian Perronne, Christine Rouzioux, Papa Salif Sow, and Virginie Ettiegne-Traoré. Trial independent data safety monitoring board: François-Xavier Blanc, Dominique Costagliola, Brigitte Autran, Ogobara Doumbo, Sinata Koula-Shiro, Souleymane Mboup, and Yazdan Yazdanpanah. ANRS representatives: Jean-François Delfraissy, Brigitte Bazin, Claire Rekacewicz, and Géraldine Colin.
Notes
Acknowledgments. We thank all patients who participated in this trial; members of the SMIT, CeDReS, CEPREF, USAC, CIRBA, CNTS, La Pierre Angulaire, Hôpital Général Abobo, Formation Sanitaire Anonkoua Kouté, Centre de santé El Rapha, the Programme PACCI team, and INSERM U897 teams (Abanou Matthieu, Aman Adou,Anasthasie Yapo, Bombo Léontine, Célestin N'chot, Christian Kouadio, Djetouan Hugues, Djobi-Djo Edouard, Goly Jocelyn, Kassi Marie-Cécile, Koffi- N'Dri Aholi, Konan Sylvie, Konaté Mamadou, Kouadio Bertin, Kouamé Martin, Kouadio Victoire, Kouakou-Aboua Adrienne, Kouakou Yao, Kouamé Antoine, Kouamé Ferdinand, Kouamé Gérald, Labibi Georgette, Lokou Benjamin, Moh Jules, N'Dri Marie Julie, Nalourgou Tuo, N'Goran Brou, Nogbout Marie-Pascale, Orne-Gliemann Joanna, Kouadio Cheftin, Ouattara Minata, Oupoh Joséphine, Sidibé Abdelh, Siloué Bertine, Soro Adidiata, Tchehy Amah-Cécile, Yao Emile, and Yao Juliette), for their valuable contributions; Gilead Sciences, for the donation of Truvada; and Merck Sharp & Dohme, for the donation of Stocrin.
Disclaimer. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the French Agence Nationale de Recherches sur le SIDA et les hépatites virales (grants 12136 and 12239).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 2.Vernazza P, Hirschel B, Bernasconi E, Flepp M. Les personnes séropositives ne souffrant d'aucune autre MST et suivant un traitement antirétroviral efficace ne transmettent pas le VIH par voie sexuelle. Bull Med Suisse. 2008 89:5, pp165–169. [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 5.Eaton JW, Johnson LF, Salomon JA, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabis F. Reality check: is the end of AIDS in sight?. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. [Google Scholar]
- 7.Dukers NH, Goudsmit J, de Wit JB, Prins M, Weverling GJ, Coutinho RA. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS Lond Engl. 2001;15:369–78. doi: 10.1097/00002030-200102160-00010. [DOI] [PubMed] [Google Scholar]
- 8.Tun W, Gange SJ, Vlahov D, Strathdee SA, Celentano DD. Increase in sexual risk behavior associated with immunologic response to highly active antiretroviral therapy among HIV-infected injection drug users. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38:1167–74. doi: 10.1086/383033. [DOI] [PubMed] [Google Scholar]
- 9.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332:605–7. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4:165–72. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS Lond Engl. 2011;25:1939–49. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;50(Suppl 3):S85–95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292:224–36. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 14.The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group. HIV treatment as prevention: models, data, and questions—towards evidence-based decision-making. PLoS Med. 2012;9:e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidance on couples HIV testing and counseling including antiretroviral therapy for treatment as prevention in serodiscordant couples. Geneva: World Health Organization; 2012. http://www.who.int/hiv/pub/guidelines/9789241501972/en/ . Accessed 11 September 2013. [PubMed] [Google Scholar]
- 16.Chemaitelly H, Shelton JD, Hallett TB, Abu-Raddad LJ. Only a fraction of new HIV infections occur within identifiable stable discordant couples in sub-Saharan Africa. AIDS. 2013;27:251–60. doi: 10.1097/QAD.0b013e32835ad459. [DOI] [PubMed] [Google Scholar]
- 17.Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2006 revision. Geneva: World Health Organization; 2006. Accessed 11 September 2013 http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf . [PubMed] [Google Scholar]
- 18.Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. Geneva: World Health Organization; 2010. http://www.who.int/hiv/pub/arv/adult2010/en/index.html . Accessed 11 September 2013. [PubMed] [Google Scholar]
- 19.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institut National de la Statistique (INS) & Ministère de la Lutte contre le Sida [Côte d'Ivoire] & ORC Macro. AIDS Indicators Survey, Côte d'Ivoire 2005. Calverton, MD: INS & ORC Macro; 2006. [Google Scholar]
- 21.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–20. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 22.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 23.Moatti J-P, Prudhomme J, Traore DC, Juillet-Amari A, Akribi HA-D, Msellati P. Access to antiretroviral treatment and sexual behaviours of HIV-infected patients aware of their serostatus in Côte d'Ivoire. AIDS Lond Engl. 2003;17(Suppl 3):S69–77. doi: 10.1097/00002030-200317003-00010. [DOI] [PubMed] [Google Scholar]
- 24.Diabaté S, Alary M, Koffi CK. Short-term increase in unsafe sexual behaviour after initiation of HAART in Côte d'Ivoire. AIDS Lond Engl. 2008;22:154–6. doi: 10.1097/QAD.0b013e3282f029e8. [DOI] [PubMed] [Google Scholar]
- 25.Protopopescu C, Marcellin F, Préau M, et al. Psychosocial correlates of inconsistent condom use among HIV-infected patients enrolled in a structured ART interruptions trial in Côte d'Ivoire: results from the TRIVACAN trial (ANRS 1269) Trop Med Int Heal TM IH. 2010;15:706–712. doi: 10.1111/j.1365-3156.2010.02524.x. [DOI] [PubMed] [Google Scholar]
- 26.Sarna A, Luchters SMF, Geibel S, et al. Sexual risk behaviour and HAART: a comparative study of HIV-infected persons on HAART and on preventive therapy in Kenya. Int J STD AIDS. 2008;19:85–9. doi: 10.1258/ijsa.2007.007097. [DOI] [PubMed] [Google Scholar]
- 27.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wamoyi J, Mbonye M, Seeley J, Birungi J, Jaffar S. Changes in sexual desires and behaviours of people living with HIV after initiation of ART: Implications for HIV prevention and health promotion. BMC Public Health. 2011;11:633. doi: 10.1186/1471-2458-11-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apondi R, Bunnell R, Ekwaru JP, et al. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS Lond Engl. 2011;25:1317–27. doi: 10.1097/QAD.0b013e328347f775. [DOI] [PubMed] [Google Scholar]
- 30.Baggaley RF, White RG, Hollingsworth TD, Boily M-C. Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology. 2013;24:110–21. doi: 10.1097/EDE.0b013e318276cad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 Serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med. 2011;8:e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean K, Anglaret X, Moh R, Lert F, Dray-Spira R. Barriers to HIV Testing in Côte d'Ivoire: The Role of Individual Characteristics and Testing Modalities. PLoS ONE. 2012;7:e41353. doi: 10.1371/journal.pone.0041353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AIM, Wensing AMJ. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 34.Moh R, Danel C, Badje A, et al. Conséquences des conflits armés sur la prise en charge des personnes infectées par le VIH: exemple de l'essai Temprano (ANRS 12136). AFRAVIH 2012–6ème Conférence Francophone VIH/SIDA; Geneva, Switzerland. 26 March 2012. [Google Scholar]
- 35.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in Rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med. 2012;9:e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delva W, Eaton JW, Meng F, et al. HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med. 2012;9:e1001258. doi: 10.1371/journal.pmed.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellan SE, Fiorella KJ, Melesse DY, Getz WM, Williams BG, Dushoff J. Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet. 2013;381:1561–9. doi: 10.1016/S0140-6736(12)61960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]