Abstract
This case report describes the outbreak of candidemia caused by non-albicans Candida (NAC) species, which within a short period of 11 days, affected six neonates housed in the same room of neonatal intensive care unit of a rural tertiary care center in Uttarakhand state, India. The NAC species isolated showed complete resistance to azole compounds tested. All the neonates were having central venous catheters at the time of diagnosis, received total parenteral nutrition and were on broad spectrum antibiotics. Though two neonates survived the infection, but four of them had an unfortunate outcome and they died despite of aggressive therapy with amphotericin B. It was concluded that candidemia was associated with previously described risk factors and that poor infection control practices were likely responsible for outbreak.
Keywords: Amphotericin B, blood stream infections, fungemia, non-albicans Candida
INTRODUCTION
Candida species have emerged as one of the most common causes of blood stream infections (BSI) among neonates and account for 9-13% of such infections, with most of the surveillance studies reporting a rising trend.[1] Although Candida albicans remains the most common fungal isolate from neonatal candidemia, longitudinal studies have detected a shift towards non-albicans Candida (NAC) species notably Candida tropicalis, Candida parapsilosis, Candida krusei and Candida glabrata.[2,3] These micro-organisms are difficult to diagnose and cause significant morbidity and mortality despite antifungal therapy.[4] The mortality associated with Candida spp. BSI is consistently high, with estimated figures between 40% and 50%.[4] A number of factors including the use of indwelling vascular devices, broad spectrum antibiotics, low birth weight (LBW), prematurity, total parenteral nutrition (TPN), gastrointestinal surgery, artificial ventilation and/or history of fungal colonization contribute to the risk.[2] Preterm very LBW (PVLBW); ≤1500 g, extremely LBW (ELBW); ≤1000 g and critically ill neonates are at highest risk of invasive Candida infections.[1]
Outbreaks of candidemia have been associated with contaminated milk bottles, parenteral nutrition, glycerin suppositories, contaminated intravenous (IV) medicines and indwelling vascular devices, syringe reutilization and also from hands of health-care workers (HCW).[5,6,7,8] Many of the other investigators did not find a common source, but outbreak was controlled after optimization of infection control policies.
Here we describe an outbreak of candidemia caused by a NAC species, which involved six neonates over a short period of 11 days in neonatal intensive care unit (NICU) of our hospital, a rural tertiary care center in Srinagar Garhwal, Uttarakhand State, India.
All the isolates were recovered in Bactec Peds plus/F culture vials of an automated blood culture system (Bactec 9120, Becton Dickinson, USA). Any growth indicated was subcultured on 5% of sheep blood agar, MacConkey's agar and Sabouraud's dextrose agar (SDA) with chloramphenicol (0.05%) and incubated at 37°C. The identification was carried out as per standard mycological techniques.[9] Briefly; the identification was done by colony morphology on SDA, chromogenic media (HiCrome Candida differential agar, Himedia Laboratories, Mumbai, India), growth at 45°C, germ tube test, chlamydospore formation, carbohydrate fermentation and assimilation tests.[9] Though the phenotypic identification tests were carried out but all the six isolates showed distinct, but identical phenotypic characters, which were not helpful in the species identification. Due to the limited laboratory capacity and resources, we were unable to determine the species of these Candida isolates and also molecular testing could not be incorporated into our investigations.
The in vitro antifungal susceptibility of all the isolates was determined by E-test (Ezy Minimum inhibitory concentration [MIC] strips, Himedia laboratories, Mumbai, India) on RPMI agar supplemented with 2% of glucose. The plates were incubated at 35°C and were read after 40 h. The isolates were considered as resistant if they exhibited the following MICs; fluconazole (FLK), ≥64 μg/ml; itraconazole (ITR), ≥1 μg/ml; amphotericin B (AMB), ≥1 μg/ml. C. albicans - American type culture collection (ATCC) 90028 and C. parapsilosis - ATCC 22019 (KWIK-STIK, Himedia Laboratories, Mumbai, India) were used as the control strains.
CASE REPORT
The present report describes six cases of neonatal candidemia among the newborns housed in the same room of a NICU at our center. The first isolation was made on the 23rd August 2012 from the blood of a preterm (gestational age 29 weeks), LBW (1200 g) neonate. The baby was in NICU for 13 days before she developed candidemia, after which she had severe respiratory distress, feed intolerance and failure to thrive. Despite aggressive treatment with AMB and FLK, she succumbed to infection within 3 days from the diagnosis. When admitted to the NICU (1st day of hospitalization), the new born did not appear to harbor infection with the Candida spp. as evidenced by the absence of yeast isolation from all the specimens taken at the time of admission. Moreover, none of the other five cases had any such infection on the day of their admittance to the same NICU room. The other five cases occurred within a period of 10 days after the patients had been in NICU for 19, 28, 8, 26 and 15 days respectively. All the patients were having central venous catheters at the time of diagnosis, received TPN and were on broad spectrum antibiotics. The characteristics of the six neonates whose blood culture yielded this NAC species are presented in Table 1. Although case 3 and case 4 survived the infection, others died despite of the treatment with AMB or liposomal AMB (AmBisome). All six cases presented more or less with the same clinical features of which most common were respiratory distress, feed intolerance, abdominal distention and failure to thrive.
Table 1.
Characteristics of neonates with candidemia
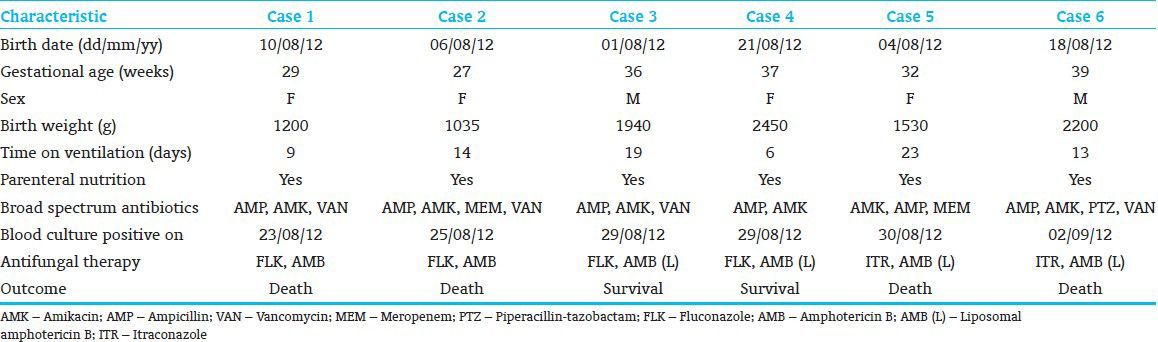
As part of the patient management strategy, the central venous catheters of the neonates were changed after the diagnosis of candidemia. Unfortunately the catheter tips were not cultured for the isolation of organism. It is therefore not clear, how much the removal of catheters contributed to the infection control, particularly in case 2 who did not responded to AMB treatment even though the yeast was sensitive to the drug in vitro.
DISCUSSION
This report is noteworthy in that it describes the emergence of diverse NAC species capable of causing outbreak of candidemia. Although C. albicans remains the most frequently isolated Candida spp. in several centers, the role of NAC species is increasing. Various outbreaks caused by NAC species in the NICU setting have been reported previously, mainly caused by C. parapsilosis and C. tropicalis.[6,7,8]
All the six neonates had central venous catheters, were on broad spectrum antibiotics and received TPN. Four neonates were premature newborns and had LBW. All these are considered as major risk factors associated with development of candidemia. Use of multiple invasive devices such as catheters, endotracheal tubes and surgery causes break in the integrity of skin/mucosa, which predisposes these sites for colonization and infection by Candida spp. Broad spectrum antibiotics ranging two-four in number were being administered to all the neonates in the present study. Antibiotics promote fungal overgrowth at the expense of normal bacterial flora and encourage translocation of yeast across the intact mucosa. The risk of candidemia is also known to increase exponentially with each class of antimicrobial used.[10] TPN induces gut mucosal atrophy and has immunosuppressive effects, which again predisposes individual for infection. PVLBW; ≤1500 g, ELBW; ≤1000 g, and critically ill neonates are at highest risk of invasive Candida infections.[1] The risk of systemic fungal infection in premature neonates weighing <1000 g is reported to be as high as 67% with a mortality rate of around 40%.[1]
Antifungal susceptibility results showed that all isolates were resistant to the azoles (FLK, ITR) tested, thus posing diagnostic and therapeutic challenges as azoles are widely used antifungals for the treatment as well as for prophylaxis of fungal infections. Resistance to azole compounds have been reported previously and is rapidly increasing particularly among NAC species.[7,10,11] None of the isolates were found resistant to AMB. With the exception of some C. lusitaniae and Candida haemulonii strains, the occurrence of AMB resistance among Candida spp. is rarely reported.[12,13] Although all the isolates were sensitive to AMB, one of the neonates (case 2) did not responded to the AMB therapy and had unfortunate outcome. The data on the antifungal susceptibilities of the isolates are depicted in Table 2.
Table 2.
Antifungal susceptibility profile of the six non-albicans Candida isolates
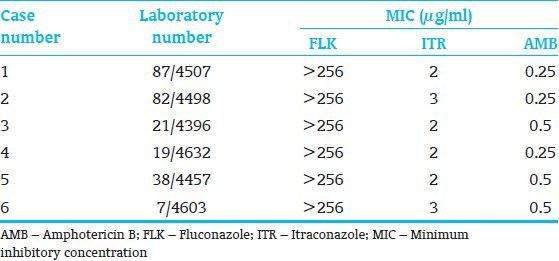
Authors speculate that horizontal transmission of the organism occurred subsequently from case one to the other five cases through direct interaction between HCW/nurses and the infants. Poor hand hygiene practices and insufficient supply of alcohol hand rubs may be the contributing factors. Several previous studies have linked the hands of HCW with Candida BSI outbreaks.[8,14] Various environmental sources like use of non-sterile cotton, overcrowding of the NICU, plastic bags for transportation of IV medicines etc., may also play vital role in transmission of the infection. The environmental sources are more likely to be problematic in resource limited settings where variety of strategies are often improvised as a result of misleading cost oriented (money saving) policies. In our center only, due to lack of the supply of sterile alcohol swabs nurses used to prepare handmade alcohol swabs with small pieces of sterile cotton kept in a non-sterile cup and an arbitrary alcohol was poured on top. These cotton swabs were used to clean the IV sites and IV ports before medication. Such improvisations should be discouraged as they may favor the outbreak resulting in significant cost as well as patient morbidity and mortality.
We believe that the Candida spp. isolated from the cases was diverse and since all the isolates were recovered over a short period of the time and exhibited nearly identical phenotypic characters, they were part of an outbreak that probably must have originated from a common source. Although no specific containment measures were taken, the outbreak subsided by strict enforcement of infection control measures. This is strong evidence in favor of outbreak control as a result of the implemented measures. Meticulous attention to hand hygiene practices, use of sterile alcohol swabs, scrupulous care of medical devices, routine glove wearing even when urgent situation arise in NICUs were the measures implemented. Unfortunately, we could not undertake epidemiological studies to trace the source of this distinct NAC species.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: Risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.Goel N, Ranjan PK, Aggarwal R, Chaudhary U, Sanjeev N. Emergence of non-albicans Candida in neonatal septicemia and antifungal susceptibility: Experience from a tertiary care center. J Lab Physicians. 2009;1:53–5. doi: 10.4103/0974-2727.59699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, Prasad K. Non-albicans Candida species in blood stream infections in a tertiary care hospital at New Delhi, India. Indian J Med Res. 2012;136:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 4.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 5.Nedret Koç A, Kocagöz S, Erdem F, Gündüz Z. Outbreak of nosocomial fungemia caused by Candida glabrata. Mycoses. 2002;45:470–5. doi: 10.1046/j.1439-0507.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, Brandt ME, et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol. 2004;42:4468–72. doi: 10.1128/JCM.42.10.4468-4472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhary A, Becker K, Fegeler W, Gugnani HC, Kapoor L, Randhawa VS, et al. An outbreak of candidemia due to Candida tropicalis in a neonatal intensive care unit. Mycoses. 2003;46:287–92. doi: 10.1046/j.1439-0507.2003.00883.x. [DOI] [PubMed] [Google Scholar]
- 8.Lupetti A, Tavanti A, Davini P, Ghelardi E, Corsini V, Merusi I, et al. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2363–9. doi: 10.1128/JCM.40.7.2363-2369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinnis MR. Laboratory Handbook of Medical Mycology. New York: Academic Press; 1980. Yeast identification; pp. 337–73. [Google Scholar]
- 10.Magill SS, Shields C, Sears CL, Choti M, Merz WG. Triazole cross-resistance among Candida spp.: Case report, occurrence among bloodstream isolates, and implications for antifungal therapy. J Clin Microbiol. 2006;44:529–35. doi: 10.1128/JCM.44.2.529-535.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giusiano G, Mangiaterra M, Garcia Saito V, Rojas F, Gómez V, Díaz MC. Fluconazole and itraconazole resistance of yeasts isolated from the bloodstream and catheters of hospitalized pediatric patients. Chemotherapy. 2006;52:254–9. doi: 10.1159/000094867. [DOI] [PubMed] [Google Scholar]
- 12.Peyron F, Favel A, Michel-Nguyen A, Gilly M, Regli P, Bolmström A. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J Clin Microbiol. 2001;39:339–42. doi: 10.1128/JCM.39.1.339-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan ZU, Al-Sweih NA, Ahmad S, Al-Kazemi N, Khan S, Joseph L, et al. Outbreak of fungemia among neonates caused by Candida haemulonii resistant to amphotericin B, itraconazole, and fluconazole. J Clin Microbiol. 2007;45:2025–7. doi: 10.1128/JCM.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YC, Lin TY, Lien RI, Chou YH, Kuo CY, Yang PH, et al. Candidaemia in special care nurseries: Comparison of albicans and parapsilosis infection. J Infect. 2000;40:171–5. doi: 10.1053/jinf.2000.0638. [DOI] [PubMed] [Google Scholar]


