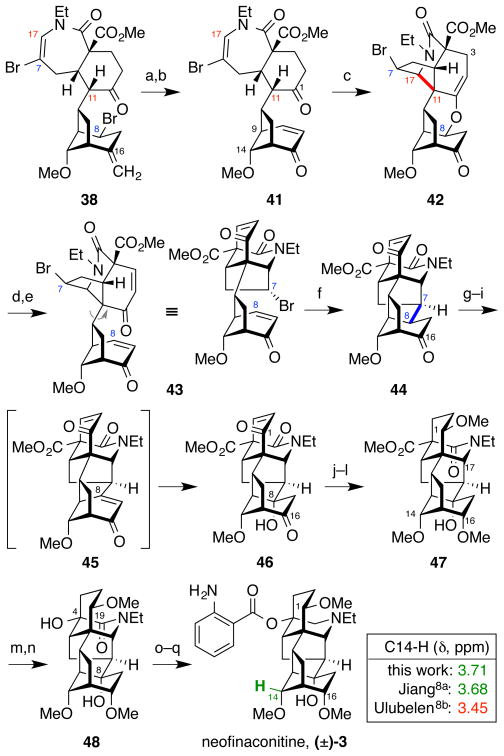
Figure 9.
Completion of the total synthesis of neofinaconitine via C11–C17 Mannich-type N-acyliminium cyclization and C7–C8 radical cyclization; diagnostic 1H-NMR peaks for neofinaconitine11a and delphicrispuline11b (CDCl3). a) OsO4, NMO, THF, H2O, then Pb(OAc)4, 65%; b) DBU, toluene, 87%; c) Tf2NH, CH2Cl2, 75%; d) CAN, CH3CN, H2O, 60 °C; e) MsCl, Et3N, CH2Cl2, 50 °C, 66% over 2 steps; f) Bu3SnH, AIBN, PhH, 80 °C, 99%; g) TMSOTf, Et3N, THF, 0 °C; h) PhSeCl, CH2Cl2, 0 °C 86% over 2 steps; i) NaIO4, THF, H2O, 59%; j) Pd/C, H2, EtOAc; k) NaBH4, MeOH, 0 °C, 89% over 2 steps; l) MeI, t-BuOK, THF, 0 °C, 34%; m) LiBH4, THF; n) CrO3, 0.5 N H2SO4, 40% over 2 steps; o) LiAlH4, THF, 85 °C; p) o-NO2BzCl, DMAP, Et3N, C6H6, 80 °C; q) Zn, HCl, MeOH, H2O, 13% over 3 steps.