Abstract
Objective
Febrile seizure (FS) as the most common form of seizures in childhood, affects 2-5% of all children across the world. The present study reviews available reports on FS recurrence frequency and evaluates its associated risk factors in Iran.
Methods
We searched the Persian database such as: SID, MagIran, Medlip, Irandoc, Iranmedex as well as English databases PubMed, ISI, and Scopus. Random effects models were used to calculate 95% confidence intervals. Meta regression was introduced to explore the heterogeneity between studies.
Findings
The overall FS recurrence rate was 20.9% [95% confidence interval (CI): 12.3-29.5%]. The frequency of FS simple and complex types was 69.3% (95% CI: 59.5-79.0) and 25.3% (95% CI: 19.6-31.0), respectively. A positive familial history of 28.8% (95% CI: 19.3-38.4%) was observed for childhood FS including 36.2% (95% CI: 27.3-39.6%) for the simple and 29.4% (95% CI: 23.1-33.5%) for the complex type. The heterogeneity of recurrent FS was significantly affected by sample size (P=0.026).
Conclusion
Almost one-third of FS children had a positive familial history. The increased risk of recurrence in patients with symptomatic seizures needs to be fully considered by parents, physicians, nurses and health policy makers.
Keywords: Febrile seizure, Recurrence, Positive familial History, Systematic review, Meta-Analysis, Iran
Introduction
Febrile seizure (FS) as the most common form of seizures in childhood, affects 2-5% of all children across the world[1, 2]. Children rarely develop their first FS before the age of 6 months or after 3 years of age[3]. The International League Against Epilepsy has defined that seizure events in infancy or childhood is featured with temperatures over 38° C without evidence of acute electrolyte imbalances and CNS infection or history of FS[4].
The direct cause of FS is unknown yet, but the most important indirect causes are reported to be fever, hypoglycemia, hypocalcemia, head injury, poisoning and drug overuse, respiratory infection or gastroenteritis[5].
There are two categories of FS including simple and complex types[6]. The simple type is characterized by an episode of generalized tonic-clonic seizure lasting less than 15 min in 24 h while in the complex type the convulsions are multiple, lasting more than 15 min. Majority (70%-75%) of FSs are of simple type[6, 7].
A positive family history for FS can be elicited in 25-40% of the patients[8]. A high concordance rate has been reported in monozygotic rather in dizygotic twins[8, 9]. The overall recurrence rate is 30%[10]. Predictors of recurrence are complex seizures, positive family history, onset at less than 12 months and temperature <40°C. If the seizure occurs soon after a fever has begun or when the temperature is relatively low, the risk of recurrence will be increased[11]. A long initial FS does not substantially boost the risk of recurrent FS, either brief or long[12]. The odds that children develop epilepsy after a FS is approximately 1%[13].
Since a systematic review and meta-analysis on FS recurrence in Iran has not yet been done, the present study reviews available reports on FS recurrence frequency and evaluates its associated risk factors in this country.
Subjects and Methods
Literature search
The search strategy, selection of publications and the reporting of results for the review was conducted in accordance with the PRISMA guidelines[14]. Literature on FS among Iranian children was acquired through searching Scientific Information Databases (SID), Global Medical Article Limberly (Medlib), Iranian Biomedical Journal (Iran Medex), Iranian Journal Database (Magiran) as well as international databases including PubMed/Medline, Scopus and ISI Web of Knowledge. The search strategy was limited to the Persian and/or English language and articles published up until Feb 2012 were considered. All publications with medical subject headings (MeSh) and keywords in title, abstract and text for words including febrile seizure were investigated. Iranian scientific databases were searched only using the keyword ‘febrile seizure’, as these databases do not distinguish synonyms from each other and do not allow sensitive search operation using linking terms such as ‘AND’, ‘OR’ or ‘NOT’. Consequently, this single keyword search was the most practical option. The seizures, pediatrics and Iran MeSh combined with the operator “OR” vs. “AND”. The search string in PubMed was ((Seizures [Title]) OR Pediatrics [Title]) AND Iran [Affiliation]).
Selection and quality assessment of articles
All identified papers were critically appraised independently by two reviewers. Disagreements between reviewers were resolved by consensus. Appraisal was guided by a checklist assessing clarity of aims and research questions. The inclusion criteria were as follow: 1) Studies in the mentioned databases with full text, despite the language of original text; 2) Hospital-based data. Exclusion criteria were 1) studies upon children overlapping time intervals of sample collection from the same origin; 2) inappropriate study design; 3) Inadequate reporting of results; 4) STROBE checklist score's below 7.75[15].
Data extraction
Data were extracted using a standardized and pre-piloted data extraction form. Data extraction was undertaken by the first reviewer and checked by a second reviewer. However, the process was discussed and piloted by both reviewers. All identified papers were critically appraised independently by both reviewers. Disagreements were resolved through discussion. Appraisal was guided by a checklist assessing clarity of aims and research questions. Information was extracted from author, title, year and setting of study, sample selection, sample size, study type, seizure types, age, STROBE score and prevalence. Therefore risk of bias as an inadequate reporting was reduced. All data-abstraction forms were reviewed and eligible papers entered into the meta-analysis.
Statistical analysis
The random effects model was used for combining results of studies in meta-analysis. Variance for each study was calculated using the binomial distribution formula. The presence of heterogeneity was determined by the Der Simonian-Laird (DL) approach[16]. Significance level was <0.1 and I2 statistic for estimates of inconsistency within the meta-analyses. The I2 statistic estimates the percent of observed between-study variability due to heterogeneity rather than to chance and ranges from 0 to 100 percent (values of 25%, 50% and 75% were considered representing low, medium and high heterogeneity respectively)[17]. A value of 0% indicates no observed heterogeneity whilst 100% indicates significant heterogeneity. For this review we determined that I2 values above 75 percent were indicative of significant heterogeneity warranting analysis with a random effect model as opposed to the fixed effect model to adjust for the observed variability. This heterogeneity was further explored through subgroup analyses and meta-regression. A univariate and multivariate approach was employed to assess the causes of heterogeneity among the selected studies. Egger test was conducted to examine potential publication bias. Data manipulation and statistical analyses were done using STATA software, version 11.2. P values <0.05 were considered as statistically significant.
Findings
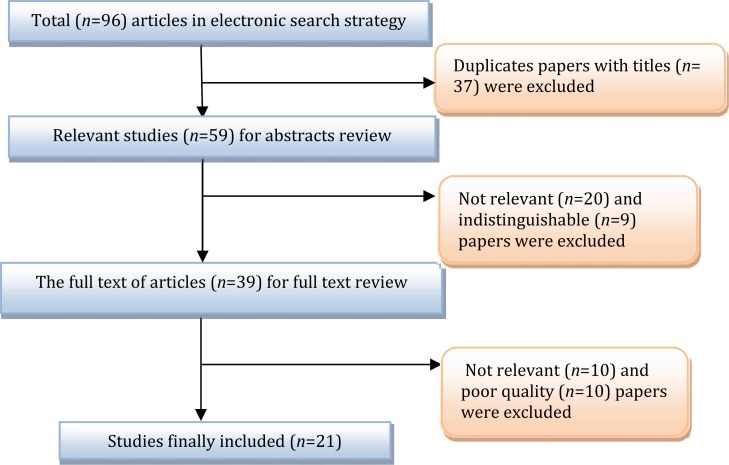
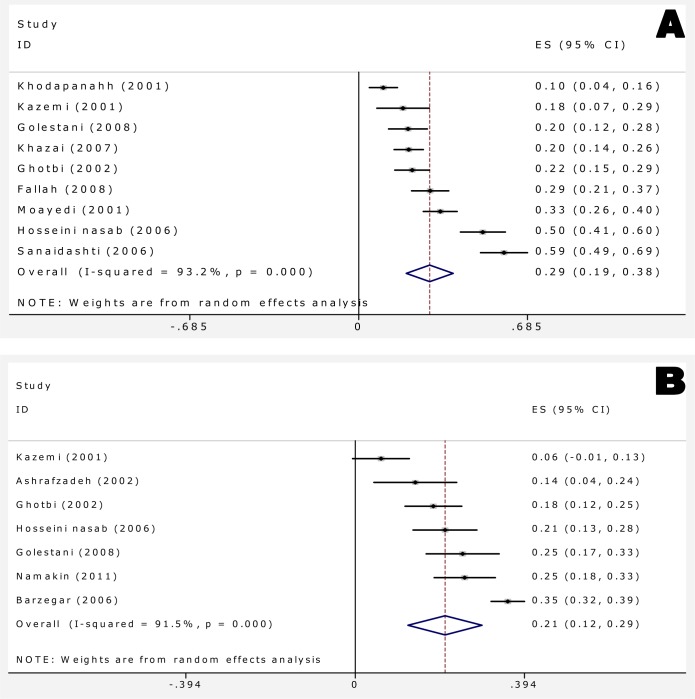
Overall, 96 studies (1 study in Pub Med, 95 studies in other databases) were identified. Of them, 75 studies were excluded based on the inclusion and exclusion criteria. Finally 21 articles including 1 in English[18] and 20 in Persian[19–38] language were adopted (Fig. 1). Looking for sample sizes, throughout the 21 selected studies, 4599 FS children (219 samples per study on average) including 2734 males (59.5%) were studied (Table 1). Age distribution and type of FSs prevalence in children under two years and 3 to 6 years were 55.8% (95%CI: 50.4–61.2) and 44.1% (95%CI: 38.8-62.2), respectively. Prevalence of simple and complex FS was 69.3% (95%CI: 59.5-79.0) and 28.3% (95%CI: 19.6-31.0), respectively (Table 2). Significant hetero-geneity was observed between studies (Q = 75.24, P≤0.001, I2=92.0%) and consequently the random effect model was employed for the meta-analysis. Recurrence and positive familial history of FS was observed in 28.8 children (95%CI: 19.3-38.4) (Fig. 2A).
Fig. 1.
Results of the systematic literature search
Table 1.
Feature of childhood febrile seizure at different regions of Iran
| Study location (city) | First author (year) | Study period | No. of patients | Gender (male) n (%) | Data collection procedure | Quality* |
|---|---|---|---|---|---|---|
| Bandar Abbas | Ahmadian (1996) [19] | 1996-1997 | 211 | 127 (0.60) | Hospital | High |
| Isfahan | Amini (2008) [20] | 2005-2007 | 1486 | 892 (0.60) | Hospital | High |
| Mashhad | Ashrafzadeh (2002) [21] | 2001-2002 | 50 | 35 (0.70) | Hospital | High |
| Tabriz | Barzegar (2006) [22] | 2001-2003 | 582 | 321 (0.55) | Hospital | High |
| Ahvaz | Dehdashtian (2008) [23] | 2003-2008 | 94 | 54 (0.57) | Hospital | High |
| Tehran | Ehsanypoor (2004) [24] | 1997-2007 | 245 | 140 (0.57) | Hospital | Medium |
| Yazd | Fallah (2008) [25] | 2004-2005 | 139 | 63 (0.55) | Hospital | High |
| Sanandaj | Ghotbi (2002) [26] | 2000-2001 | 115 | 70 (0.61) | Hospital | Medium |
| Yazd | Golestan (2008) [27] | 2002-2005 | 100 | 59 (0.59) | Hospital | High |
| Tehran | Hassanpoor (2009) [28] | 2003-2005 | 103 | 64 (0.62) | Hospital | High |
| Kerman | Hosseininasab (2006) [29] | 2000-2002 | 115 | 68 (0.59) | Hospital | High |
| Zanjan | Kazemi (2001) [30] | 2000-2001 | 50 | 33 (0.66) | Hospital | High |
| Zahedan | Khazai (2007) [31] | 2005-2006 | 178 | 94 (0.53) | Hospital | High |
| Tehran | Khodapanahandeh (2001)[32] | 2007-2008 | 107 | 64.60) | Hospital | High |
| Bandar Abbas | Moayedi (2001) [33] | 2001-2002 | 181 | 112 (0.62) | Hospital | Medium |
| Ilam | Mohammadi (2008) [34] | 2007-2008 | 172 | 98 (0.57) | Hospital | High |
| Birjand | Namakin (2011) [35] | 2006-2007 | 145 | 84 (0.61) | Hospital | High |
| Babel | Rasholi (1999) [36] | 1999-2000 | 230 | 138 (0.60) | Hospital | High |
| Zanjan | Sadeghzadeh (2011) [37] | 2005-2006 | 117 | 64 (0.55) | Hospital | High |
| Bushehr | Sanaidashti (2006) [38] | 2005-2006 | 102 | 64 (0.65) | Hospital | Low |
| Kashan | Talebian (2006) [42] | 2001-2002 | 120 | 72 (0.60) | Hospital | Medium |
Score of STROBE checklist. Under 7.75 = excluded; among 7.75 to 15.5: low; among 15.5 to 23.5: medium; higher than 23.5: High
Table 2.
Prevalence of febrile seizure among Iranian children through random effect models
| Variables | No. of studies | No. of Patients | Prevalence% (95% CI) | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | P value | ||||
| Simple seizures | 13 | 1859 | 69.3(59.5-79.0) | 96.5 | <0.001 |
| complex seizures | 12 | 1809 | 25.3(19.6-31.0) | 88.8 | <0.001 |
| Less than two years | 9 | 1587 | 55.8(50.4-61.2) | 77.0 | <0.001 |
| Tree to six years | 9 | 1456 | 44.2(38.8-49.6) | 77.0 | <0.001 |
CI: Confidence Interval
Fig. 2.
Forest plots of positive family history (A) and recurrent febrile seizure (B) for random effects meta-analyses (Squares represent effect estimates of individual studies with their 95%confidence interval (CI) of positive family history with size of squares proportional to the weight assigned to the study in the meta-analysis. The diamond represents the overall result and 95%CI of the random-effects meta-analysis).
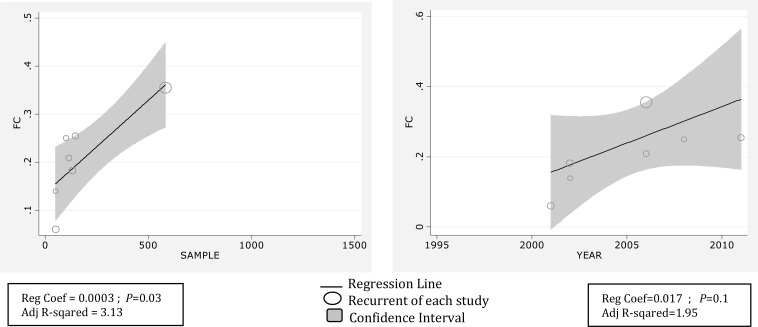
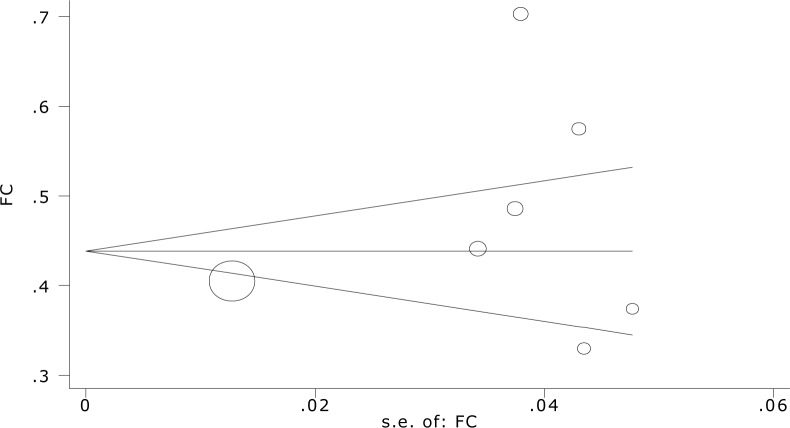
Overall recurrence rate of FS was 20.9 percent with (95%CI: 12.3-29.5) (Fig. 2B). Meta-regression showed association between year of study and prevalence rate of recurrent FS, it showed also causes of the variability in the results of studies. Meta-regression showed variability in prevalence of recurrent FS a non-significant effect for years (Reg Coef = 0.017, P=0.1). Fig. 3 shows the prevalence rate of recurrent FS that has had an increasing trend during 1995 till 2010. Meta-regression analysis found that sample size significantly affects heterogeneity for the factor ‘recurrent rate FS’ (Reg Coef =0.0003, P=0.03) (Fig. 3). Meta-regression showed that one reason of variability among studies is sample size; studies with large sample size show higher prevalence rate of recurrent FS in comparison to studies with small sample size. Publication bias is a bias with regard to what is likely to be published, among what is available to be published. Publication bias is the term for what occurs whenever the research that appears in the published literature is systematically unrepresentative of the population of completed studies. There was no evidence of publication bias (Egger's test β0: 0.04; P=0.96) (Fig. 4), so we tried to consider most of published articles in this subject.
Fig. 3.
Meta-regression plots of change in FS recurrence according to changes in continuous study moderator's year and sample size
Fig. 4.
Begg's funnel plot (pseudo 95%confidence limits) showing mean difference in recurrence of febrile Seizure by standard error of mean difference
Discussion
The present review aimed to provide recurrent rate and predictive risk factors that enhance risk of recurrence in Iranian children using the Persian and/or English language articles published up to Feb 2012. This study shows that the pool recurrence FS among Iranian children was (20.9%). Previous review studies have reported (Berg, 1990; Offringa, 1994) this as being 31.8% and 32%, respectively[2, 10]. Berg (2008) has reported recurrence rate of 40-50% in untreated children[39]. A possible explanation for this inconsistency might be due to the fact that our result was hospital-based and patients were treated. Therefore, treatment process may reduce this risk. Meta-regression thus helps exploring several possible reasons for the observed heterogeneity among studies. Results of the meta-regression found no statistically significant relation between year variations in recurrence of FS which shows an approximately constant recurrence during study period. Results of the meta-regression also found that recurrence of FS and sample size produced significant levels of heterogeneity. As a result, increasing the sample size is associated with increased recurrence (Fig. 4). Younger age at onset is important predictor of recurrent FSs and also increases the risk of later FS[43]. Peak of FS episode lies in the first year of life. Thereafter incidence declines with increasing age[3]. 58.8% of children with FS were younger than 24 months in this study. These findings correlate with study conducted by Shi XL et al instudy 2012, Most (75.7%) children experienced their first onset of FS at 6 months to 3 years of age (median: 16 months), 39.5% of cases were below 12 months and 60.50% over 12 months[40].
A positive family history of FS reveals the importance of genetic factors and common environmental exposures[9]. Berg (2008) found that family history was not consistently associated with an increased risk of FS[39]. In the present study, 28.8% of children had a positive family history of FS in agreement with Eseigbe (2012) findings which showed a prevalence rate of 29.4%[8]. Tosune (2011) reported that 57% of children with FS had a positive family history[41]. Other studies have also reported similar results confirming that positive family history increases risk of FS significantly[2, 40, 41]. The positive family history for the simple and complex type was 36.2% and 29.4%, respectively which is consistent with other reports[6].
There are some limitations in the present study which need to be addressed. It was an observational study and patients were not randomly selected. Therefore selection bias and confounding seem to be expected. Meanwhile, the authors’ ability to assess the quality of studies was limited by the fact that many studies failed to offer detailed information of selected subjects or valid data on important factors.
Conclusion
Our analysis suggests the need for large population-based incidence studies of febrile seizure, particularly in children aged under six year, to generate more accurate estimates as well as provide a reasonably robust assessment of heterogeneity. Almost one-third of FS children had a positive familial history. The increased risk of recurrence in patients with symptomatic seizures needs to be fully considered by parents, physicians, nurses and health policy makers.
Conflict of Interest
None
References
- 1.Sampaio LP, Caboclo LO, Kuramoto K, et al. Prevalence of Epilepsy in Children From a Brazilian Area of High Deprivation. Pediatr Neurol. 2010;42(2):111–7. doi: 10.1016/j.pediatrneurol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Berg A, Shinnar S, Hauser W, et al. Predictors of recurrent febrile seizures: a metaanalytic review. J Pediater. 1990;116(3):329–37. doi: 10.1016/s0022-3476(05)82816-1. [DOI] [PubMed] [Google Scholar]
- 3.van Zeijl JH, Mullaart RA, Borm GF, et al. Recurrence of febrile seizures in the respiratory season is associated with influenza A. J Pediatr. 2004;145(6):800–5. doi: 10.1016/j.jpeds.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 4.Oka E, Ishida S, Ohtsuka Y, et al. Neuroepidemiological Study of Childhood Epilepsy by Application of International Classification of Epilepsies and Epileptic Syndromes (ILAE, 1989) Epilepsia. 1995;36(7):658–61. doi: 10.1111/j.1528-1157.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 5.Pavlidou E, Tzitiridou M, Kontopoulos E, et al. Which factors determine febrile seizure recurrence? A prospective study. Brain Dev. 2008;30(1):7–13. doi: 10.1016/j.braindev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89(8):751–6. doi: 10.1136/adc.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugai K. Current management of febrile seizures in Japan: An overview. Brain Dev. 2010;32(1):64–70. doi: 10.1016/j.braindev.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Eseigbe E, Eseigbe P, Adama S. Febrile seizures in Kaduna, north western Nigeria. Niger Med J. 2012;53(3):140–4. doi: 10.4103/0300-1652.104383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuyama Y, Seki T, Ohtsuka C, et al. Practical guidelines for physicians in the management of febrile seizures. Brain Dev. 1996;18(6):479–84. doi: 10.1016/s0387-7604(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 10.Offringa M, Bossuyt PMM, Lubsen J, et al. Risk factors for seizure recurrence in children with febrile seizures: A pooled analysis of individual patient data from five studies. J Pediatr. 1994;124(4):574–84. doi: 10.1016/s0022-3476(05)83136-1. [DOI] [PubMed] [Google Scholar]
- 11.Bettis DB, Ater SB. Febrile seizures: Emergency department diagnosis and treatment. J Emerg Med. 1985;2(5):341–8. doi: 10.1016/0736-4679(85)90287-2. [DOI] [PubMed] [Google Scholar]
- 12.Siqueira LF. Febrile seizures: update on diagnosis and management. Rev Assoc Med Bras. 2010;56(4):489–92. doi: 10.1590/s0104-42302010000400026. [In English, Portuguese] [DOI] [PubMed] [Google Scholar]
- 13.Baldin E, Ludvigsson P, Mixa O, et al. Prevalence of recurrent symptoms and their association with epilepsy and febrile seizure in school-aged children: A community-based survey in Iceland. Epilepsy Behav. 2012;23(3):315–9. doi: 10.1016/j.yebeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Grossman P, Niemann L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Der Simonian R, Laird N. Meta-analysis in clinical trials. Controll Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Ades AE, Lu G, Higgins JP. The Interpretation of Random-Effects Meta-Analysis in Decision Models. Med Decis Making. 2005;25(6):646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 18.Fallah R, Karbasi S. Recurrence of febrile seizure in Yazd, Iran. Turk J Pediatr. 2010;52(6):618–22. [PubMed] [Google Scholar]
- 19.Ahmadian M, Javadi N. Etiologic factors and risk factors in 211 patients with seizure. Hormozgan Med J. 1996;12(2):145–8. [In Persian] [Google Scholar]
- 20.Amini A, Kazemi A, Ghorbani A. Causes of Seizures in Children. Iran J Neurol. 2008;7(24):355–60. [In Persian] [Google Scholar]
- 21.Ashrafzadeh F, Hashemzadeh A, Malek A. Febrile seizure in children six months to six years. Iran J Otorhinolaryngol. 2001;16(1):33–9. [In Persian] [Google Scholar]
- 22.Barzegar M, Karegar mahe M, Kivancheh N. Epidemiologic and clinical characteristics of seizures in children with first febrile attack. Med J Tabriz Univ Med Sci. 2006;28(1):17–21. [In Persian] [Google Scholar]
- 23.Dehdashtian M, Momen AA, Ziae T, et al. Evaluation of seizure etiology in convulsive neonates admitted to Imam Khomeini and Abozar hospitals of Ahvaz 2004-2007. Jundishapur Sci Med J. 2009;8(2):163–7. [In Persian] [Google Scholar]
- 24.Ehsanypoor F, Khdapanahandeh F, Aslani Z. The frequency of meningitis in children hospitalized with febrile seizures. Iran J Med Sci. 2004;11(44):907–12. [In Persian] [Google Scholar]
- 25.Fallah R, Akhavan-Krbasi S, Mir-Nasseri F. Demographic and clinical characteristic children with first febrile seizure. J Shahid Sadoughi Uni Med Sci. 2008;16(5):65–1. [In Persian] [Google Scholar]
- 26.Ghotbi N, Soleimani S. Causes of seizures in children 1 month to 12 years. Sci J Kurdistan Univ Med Sci. 2002;7(25):32–6. [In Persian] [Google Scholar]
- 27.Golestan M, Fallah R, Akhavan Krbasi S. Hundred cases of cerebrospinal fluid in children hospitalized due to febrile seizures. J Shahid Sadoughi Uni Med Sci. 2008;16(5):3–7. [In Persian] [Google Scholar]
- 28.Hassanpoor H, Ghofrani M, Taheri N, et al. Risk factors in the recurrence of seizures with fever. J Iran Univ Med Sci. 2009;16(65):46–54. [In Persian] [Google Scholar]
- 29.Hosseininasab A, Daiparizzi MH, Alidosti K. Demographic characteristics and risk factors of febrile seizure. J Med Counc IR Iran. 2006;24(2):107–12. [Google Scholar]
- 30.Kazemi A, Mousavi Nasab N, Fatemi K. Cerebrospinal fluid examination in children hospitalized. J Zanjan Univ Med Sci. 2001;9(35):32–6. [In Persian] [Google Scholar]
- 31.Khazai T, Hossein Zadeh A, Javadzadeh M. Causes of seizures in children. J Birjand Univ Med Sci. 2007;14(4):45–52. [In Persian] [Google Scholar]
- 32.Khodapanahandeh F. Studied 107 children with febrile seizures. J Iran Univ Med Sci. 2001;8(25):175–8. [In Persian] [Google Scholar]
- 33.Moayedi AR, Nazemi Qshmy A, Safdarian F. Epidemiology and etiology of seizures and fever in children. Hormozgan Med J. 2005;9(3):153–6. [In Persian] [Google Scholar]
- 34.Mohammadi J. Biochemical Disorders in Children with Febrile Seizure. J Ilam Univ Med Sci. 2008;16(4):1–6. [In Persian] [Google Scholar]
- 35.Namakin K, Shryfzadh G, Rezaei S. Demographic and clinical features febrile seizures. J Birjand Univ Med Sci. 2010;17(4):281–7. [In Persian] [Google Scholar]
- 36.Rasoli M, Moghimian A. Causes of fever in children with febrile seizures in children's hospital. J Babol Univ Med Sci. 1999;10(3):131–7. [In Persian] [Google Scholar]
- 37.Sadeghzadeh M, Asl P, Mousavi-Nasab N, et al. Relationship between serum zinc levels and febrile seizure. J Zanjan Univ Med Sci. 2011;19(74):17–24. [Google Scholar]
- 38.Sanaidashti A, Karam A, Pazaki R. Risk factors for febrile seizures. Iran South Med J. 2006;9(2):168–74. [In Persian] [Google Scholar]
- 39.Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49(Suppl 1):13–8. doi: 10.1111/j.1528-1167.2008.01444.x. [DOI] [PubMed] [Google Scholar]
- 40.Shi X, Lin Z, Ye X, et al. An epidemiological survey of febrile convulsions among pupils in the Wenzhou region. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(2):128–30. [In Chinese] [PubMed] [Google Scholar]
- 41.Tosun A, Koturoglu G, Serdaroglu G, et al. Ratios of nine risk factors in children with recurrent febrile seizures. Pediatr Neurol. 2010;43(3):177–82. doi: 10.1016/j.pediatrneurol.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Talebian A, Momtazmanesh N, Musavi GA, et al. Relationship between febrile seizure and anemia. Iran J Pediatr. 2005;16(1):79–82. [In Persian] [Google Scholar]
- 43.Mohammadi M. Febrile seizures: four steps algorithmic clinical approach. Iran J Pediatr. 2010;20(1):5–15. [PMC free article] [PubMed] [Google Scholar]