Abstract
The goal for current orthopedic implant research is to design implants that have not only good biocompatibility but also antibacterial properties. TiO2 nanotubes (NTs) were fabricated on the titanium surface through electrochemical anodization, which added new properties, such as enhanced biocompatibility and potential utility as drug nanoreservoirs. The aim of the present study was to investigate the antibacterial properties and biocompatibility of NTs loaded with vancomycin (NT-V), both in vitro and in vivo. Staphylococcus aureus was used to study the antibacterial properties of the NT-V. There were three study groups: the commercially pure titanium (Cp-Ti) group, the NT group (nonloaded vancomycin), and the NT-V group. We compared NT-V biocompatibility and antibacterial efficacy with those of the NT and Cp-Ti groups. Compared with Cp-Ti, NT-V showed good antibacterial effect both in vitro and in vivo. Although the NTs reduced the surface bacterial adhesion in vitro, implant infection still developed in in vivo studies. Furthermore, the results also revealed that both NTs and NT-V showed good biocompatibility. Therefore, the NTs loaded with antibiotic might be potentially used for future orthopedic implants.
Keywords: TiO2 nanotubes, biocompatibility, antibacterial properties, osteoblasts, vancomycin
Introduction
The number of patients with a need for orthopedic implants, such as internal fixation, external fixation, and prostheses, has increased rapidly over the past few years. Infection and poor osseointegration are two of the main causes of implant failure. Despite strict sterilization procedures, infection rates have been reported to be 1%–4% in primary total joint replacement.1,2 Furthermore, the rate of implant infection can be as high as 50% when the bone is fixed with external fixators.3 Therefore, there is a tremendous need for improvement of antimicrobial prophylaxis in orthopedic implants. In general, antimicrobial prophylaxis could be any measure taken in order to prevent implant infection.
Bacterial adhesion is known to be the first and most important step in biofilm formation.4,5 Once biofilms have formed on the implants, they can be very difficult to treat.6,7 Preventing bacterial adhesion plays a key role in preventing implant infection.4,5 Current common strategies for infection prevention and treatment include systemic antibiotic treatment and the use of antibiotic-loaded cement; however, systematic drug treatment entails several drawbacks, such as poor bioavailability, low efficacy, lack of selectivity, and toxicity.8 Local delivery of antibiotics is thought to be safer and more effective compared with systematic administration.9–11 The use of bone cement loaded with antibiotics has become increasingly adopted in the management of infected joint arthroplasty and infection prophylaxis in primary joint replacement.11 However, the use of cementless orthopedic implants has increased over the years, especially in relatively young patients.12 Unfortunately, orthopedic cementless implants have not been established yet for the purpose of infection prophylaxis through the use of local antibiotics.
Titanium and its alloys are widely used in orthopedic implants because of their good biocompatibility and mechanical properties. The oxide layer (mainly TiO2) is spontaneously formed at its surface when exposed to air.13 However, the native TiO2 layer is bioinert,13 and the lack of juxtaposed bone osseointegration into titanium might lead to implant failure. Recent studies have demonstrated that altering the titanium implant-surface topography leads to bone cell-function improvement.14–22 TiO2 nanotube (NT) layers fabricated on the surface of the titanium by electrochemical anodization have received considerable attention in orthopedic research, due to their increased osseointegration compared to that of unanodized titanium.15,18,22 Furthermore, conventional titanium could be transformed into a novel drug-eluting nanotubular titanium.23,24 Recently, Ercan et al reported that NTs showed robust antibacterial properties in vitro.25 However, the in vivo antibacterial efficacy of the NTs is generally unknown. Popat et al reported that NTs loaded with gentamicin decreased bacterial adhesion and improved osteoblast function in vitro.24 However, to our knowledge, there has been no study to report the in vivo antimicrobial properties of NTs loaded with antibiotics.
The primary purpose of our study was to evaluate the antibacterial ability of TiO2 nanotubes loaded with vancomycin (NT-V) in vitro and in vivo. The secondary purpose was to evaluate the biocompatibility of the NT-V, and the third purpose was to evaluate the antibacterial ability of the NTs in in vitro and in vivo environments.
Materials and methods
Fabrication of TiO2 nanotubes on implants
The protocol for the preparation of NTs on titanium by anodization was followed as previously described.26 In brief, the NTs were fabricated on the titanium substrates (Alfa Aesar, Ward Hill, MA, USA; 99.8% pure) by using an electrolyte with 0.5 vol% hydrofluoric acid at 20 V for 30 minutes. A platinum electrode served as the cathode. The specimen was cleaned using deionized water, and the samples were sintered at 500°C for 2 hours. The surface morphologies of the NTs were studied by scanning electron microscopy (SEM) (S-3400; Hitachi, Tokyo, Japan). The NTs used for this study were about 80 nm in diameter and 800 nm in length (Figure 1A). For in vitro studies, the samples were made into slices (1 × 1 cm). For in vivo studies, the implant rods (1 mm in diameter and 20 mm in height) were implanted into the femur of Sprague Dawley (SD) rats (Figure 2A–C). All the samples used for cellular research and animal experiments were sterilized by ethylene oxide using an ethylene oxide sterilizer (ZMW-300 L; Southern Medical Equipment, Guangzhou, People’s Republic of China) before use.
Figure 1.
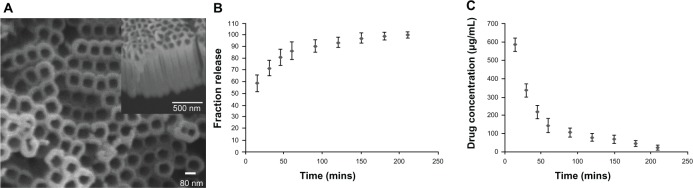
(A–C) TiO2 nanotubes and drug release. (A) Scanning electron microscopy (SEM) images of TiO2 nanotubes (top and cross-section views). The SEM image shows pores instead of nanotube structures, with pore diameters of about 80 nm and a length of about 800 nm. (B and C) Release kinetics of vancomycin loaded to the TiO2 nanotube Titanium (Ti) samples.
Figure 2.
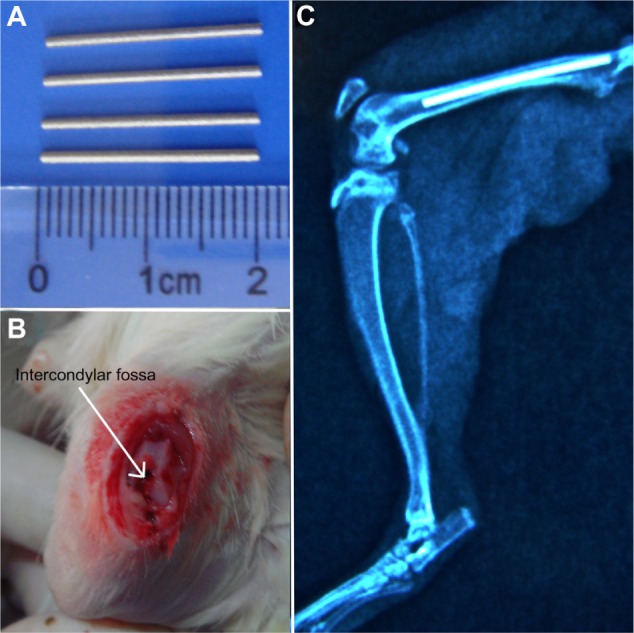
(A–C) Animal model (Sprague Dawley rats). (A and B) The Titanium (Ti) rods were implanted in the femur through the hole in the femoral intercondylar fossa; (C) postoperative X-ray with the implanted Ti rods in the femur.
Drug loading and release from the nanotubes
The vancomycin (Sigma-Aldrich, St Louis, MO, USA) was loaded into the NTs by a simplified lyophilization method. In brief, 1,000 mg of vancomycin was dissolved in 10 mL of phosphate-buffered saline (PBS; Sigma) solution (100 mg/mL). The surface of the NTs was cleaned with deionized water before vancomycin loading. Then, 20 μL of the drug solution was pipetted onto the implant surface. The implants were gently shaken to evenly spread the drug solution on the surface. The materials were dried for 1 hour at room temperature. After drying, the loading step was repeated. The surface vancomycin concentration was adjusted to 500 μg/cm2. The protocol to release the drug from the NTs was followed as previously described.24,27 In brief, the surface was immersed in 500 μL of PBS in a twelve-well plate (Corning, Corning, NY, USA) at room temperature with orbital shaking of 70 rpm. A 200 μL sample was taken after specific time intervals to determine the release kinetics. The solution was restored to 500 μL by the addition of 200 μL of fresh PBS each time. The extract was analyzed for drug concentration using an ultraviolet spectrophotometer (Nano-Photometer®; Implen, Munich, Germany) at 237 nm. A standard curve was created for vancomycin concentration using certain known vancomycin concentrations. The vancomycin concentration in the PBS was then determined based on the standard curve.
Bacterial culture
Antibacterial ability was evaluated using Staphylococcus aureus (29213; American Type Culture Collection, Manassas, VA, USA). The vancomycin minimal inhibitory concentration (MIC) for S. aureus was 0.5 μg/mL in this study. The S. aureus was cultured in Luria–Bertani (LB) agar plates (Corning). Before bacterial inoculation, the S. aureus was prepared in LB medium at 37°C for 24 hours. The bacterial concentration was adjusted to a final density of 107 colony-forming units (CFU)/mL for the in vitro study. However, it was adjusted to a final density of 108 CFU/mL for the in vivo study. Bacterial concentration was assessed via a simple optical density measurement.
Antibacterial effect of NTs in vitro
Bacterial adhesion
Bacterial adhesion was investigated on commercially pure titanium (Cp-Ti), NTs, and NT-V using a live/dead backlight bacterial viability kit (Syto-9 and propidium iodide [PI]) (Life Technologies, Carlsbad, CA, USA) and SEM (S-3400; Hitachi). For the in vitro study, the bacteria were resuspended to a final density of 107 CFU/mL. Each sample was cultured in 1 mL of this bacteria suspension in LB medium (Sigma-Aldrich) at 37°C for 2 and 6 hours. At the end of the incubation period, the substrates were washed three times with 3 mL of sterile PBS. Half of the substrates were stained with Syt 0–9 and PI stain for 15 minutes in the dark. The samples were visualized and analyzed for the live/dead bacterial ratio under a confocal laser-scanning microscope (CLSM) (Fv10i; Olympus, Tokyo, Japan). The living cells appeared in green, while the dead cells appeared in red. Bacterial cell counts were completed in a 200 × 200 μm field of view and were repeated three times for each sample. The difference in the numbers of dead bacteria on each sample was statistically analyzed. Furthermore, other samples were also observed with SEM. The samples were fixed in 2.5% glutaraldehyde for 1 hour, followed by a PBS wash. The samples were then dehydrated in serial concentrations of ethanol: 75%, 80%, 90%, 95%, and 100% (15 minutes each). All the samples were further dried with a critical point dryer and were sputter-coated with gold. They were then observed under SEM at an accelerating voltage of 10 kV.
Microbiological evaluation (planktonic bacterial viability)
The brain–heart infusion (BHI) agar plates were used to determine planktonic bacterial cell vitality. A total of 100 μL of each culture medium was placed into BHI agar plates after being cultured with metal materials and was incubated at 37°C for 24 hours to determine the number of bacteria alive.
In vivo study design
The in vivo study consisted of three groups with a total of 36 SD rats. Each group consisted of 12 animals. All the experiments were carried out in conformity with the guidelines on the protection of animals used in experiments. A total of 36 3-month-old male SD rats (350–400 g each) were used for this study. The SD rats were obtained from the Laboratory Animal Holding Unit, China Medical University. For the in vivo study, the bacterial density was diluted to 108 CFU/mL. Each inoculation volume was 100 μL for the in vivo infection study, which was equivalent to 107 CFU. The rats were randomly divided into three groups and were implanted with the bacteria-contaminated rods in their femoral canal. These groups included 1) 12 rats that were implanted with Cp-Ti rods contaminated with bacteria, 2) 12 rats that were implanted with NT rods contaminated with bacteria, and 3) 12 rats that were implanted with NT-V rods contaminated with bacteria. All the animals were killed after 30 days, and histological and microbiological methods were used to detect the infection. The study and its design were approved by the local animal committee (First Affiliated Hospital of Chinese Medical University, Shenyang, People’s Republic of China).
Surgical procedures
Prior to the surgery, animals were anesthetized with 10 mg/kg of xylazine (Fujian Fukang Pharmaceutical, Fuzhou, People’s Republic of China) and 80 mg/kg of ketamine (Fujian Fukang Pharmaceutical) injection. Following anesthesia, the skin was shaved and cleaned with povidone iodine. The intercondylar fossa of the femur, which was the deep notch between the medial and lateral femoral condyle (Figure 2B), was approached via a 1 cm anteromedial incision, followed by dislocation of the patellar tendon (Figure 2A). A hole was drilled into the femoral intercondylar fossa with a 2 mm Kirschner wire (Figure 2B). The bacteria were injected into the femoral canal at the same time as the materials’ implantation. The S. aureus bacterial suspension (107 CFU/100 μL) was introduced into the femoral canal through the hole in the femoral intercondylar fossa. A 16 G needle was inserted into the femoral canal next to the rods, and a total of 100 μL of the S. aureus suspension (107 CFU) was introduced into the femoral canal. After the implantation and bacterial inoculum, the hole in the intercondylar fossa was sealed with bone wax. The fascia and subcutaneous layers were closed, and a postoperative X-ray (Figure 2C) was performed with a mobile X-ray machine (Mobilett XP Hybrid; Siemens, Munich, Germany). Animals were killed after 30 days following the implantation by administration of an overdose of xylazine and ketamine.
Clinical assessment
Thirty days following the surgery, the rats were killed and the femurs were longitudinally cut into two parts. During the dissection in order to harvest the bone, the soft tissue and the intramedullary cavity were assessed for pus and abscess formation on the femur.
Microbiological evaluation
The LB agar plates were used for microbiological evaluation. The infection was determined based on the positive bacterial culture growth on the plates. The rods were carefully removed from the femoral canal under sterile conditions. The rods were rolled out over the plates. Swabs were taken from the tissues surrounding the rods. The swabs were weighed and subsequently ground in PBS. The swabs were then placed into the LB plates, which were incubated at 37°C for 24 hours. The number of CFUs was counted on each plate and expressed as CFU/mg. The average of all three dilutions was calculated to obtain the number of CFUs for each sample.
Cell culture
Mouse osteoblasts (MC3T3-E1, CRL-2593) were cultured in α-medium with 10% fetal bovine serum (Life Technologies) and 1% penicillin/streptomycin (HyClone; Thermo Fisher Scientific, Logan, UT, USA) in a humidified atmosphere at 37°C in 5% CO2. The culture medium was replaced every other day, and the cells were passaged once at 80% confluence. The osteoblasts were seeded onto Ti, NT, and NT-V substrates at an initial density of 104 cells/cm2.
Cell adhesion and proliferation
The osteoblasts were seeded (104 cells/cm2) on the sample surface. Osteoblast adhesion was investigated at 6 and 24 hours, and cell proliferation was investigated on days 3 and 5. The cells were stained with 4′,6-diamidino-2-phenylindole (Life Technologies) and were counted under the CLSM. Cell proliferation was also determined on the materials (Cp-Ti, NTs, and NT-V) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Life Technologies) assay. The MC3T3-E1 cells were cultured onto the bare Ti, NT, NT-V substrates at an initial density of 1 × 104 cells/cm2. After being cultured for 1 and 3 days, the samples were washed in PBS and transferred to a new 12-well cell-culture plate for analysis. The MTT solution (1 mL) was added to each well and was incubated at 37°C for 4 hours in a 5% CO2 incubator. The MTT solution was discarded, and 1 mL dimethyl sulfoxide was added into each well and pipetted up and down several times in order to make sure the formed formazan dyes were completely dissolved. Optical density was measured at the wavelength of 570 nm by a spectrophotometer (Spectramax 384 Plus; Life Technologies).
Cell-morphology observation
The osteoblasts were seeded onto Ti, NT, and NT-V substrates at an initial density of 104 cells/cm2. The osteoblast cells were observed on the implant on days 1 and 3 with SEM. SEM preparation was previously described in this paper.
Cell viability
Cell viability was determined by acridine orange/ethidium bromide (AO/EB) assay. The osteoblasts were cultured onto the bare Ti, NT, and NT-V substrates at an initial density of 2 × 104 cells/cm2. After 12 and 24 hours, the samples were washed in PBS. The living and dead cells were stained with AO/EB at room temperature and observed on the CLSM.
Statistical analysis
Statistical analysis was performed using Student’s t-test using SPSS 13.0 software (IBM, Armonk, NY, USA). The hypothesis was that vancomycin coating could reduce infection rates by 50% and would be deemed to have statistical significance in the vivo study. Statistical significance was set at P<0.05.
Results and discussion
Bacterial adhesion is the first and most important step of implant infection.4,5 Preventing bacterial adhesion could be an attractive way to prevent implant infection. Ercan et al25 reported that NTs had robust in vitro antibacterial ability, and demonstrated that NTs with larger diameter (80 nm) had better antibacterial ability than those with smaller diameter in vitro. Popat et al loaded NTs with gentamicin, showed that nanotubular features could be effectively filled with the drug, and significantly reduced Staphylococcus epidermidis adhesion.24 However, there have been no previous in vivo studies reporting on NTs loaded with antibiotics. Therefore, the present study focused on assessing the antibacterial ability of NTs (80 nm diameter) and NT-V both in vivo and in vitro. Vancomycin was chosen as the loading drug for several reasons. First, it is effective against most kinds of bacteria, such as methicillin-resistant S. aureus and methicillin-resistant S. epidermidis.28 Second, a toxic dose of vancomycin is up to 1,000 μg/mL, which is relatively low compared with other commonly used antibiotics, such as gentamicin, tobramycin, and rifampicin.29,30 Ideally, the drug should not have interfered with the cellular processes if the drug was released at physiologically relevant rates. The dosage of the vancomycin in this study was 500 μg/cm2.
Fabrication of the TiO2 nanotubes
Figure 1A shows an SEM image of NTs (diameter of 80 nm and length of 800 nm), which were fabricated on the titanium surface using an anodization voltage of 20 V for 30 minutes. The NTs were vertical and were uniformly distributed over the titanium. It is well known that the diameter and length of NTs can be precisely controlled by varying the anodization voltage and time.15–21 In this study, we investigated the antibacterial ability and biocompatibility of the NTs (80 nm in diameter and 800 nm in length), which were filled with vancomycin in vitro and in vivo.
Drug loading and release
Figure 1B shows the release data obtained from the NTs (80 nm in diameter and 800 nm in length). As expected, the NT drug release could be divided into two parts: initial burst release and relatively slow release. Other reports have shown similar release times.24,27 Figure 1C shows concentrations of approximately 600 μg/mL at 15 minutes, 142.5 μg/mL at 60 minutes, and 45 μg/mL at 180 minutes in vitro, which were much higher than that of the MIC of the S. aureus in this study (0.5 μg/mL). The 210-minute release of vancomycin mainly enhanced initial antibacterial efficacy. Rapid drug release from the implants inhibited bacterial invasion and prevented early contamination of the implant site. About 58% of the vancomycin was released from the NTs in the first 15 minutes, and the relative release was completed over the following 180 minutes. However, a drug-release model can hardly concisely reflect in vivo drug-release kinetics. The implant was placed in the femoral canal, which was mainly surrounded by blood or hematoma (such as the femoral component of prosthesis in the total hip arthroplasty) in a closed environment. The hematoma, a localized collection of blood outside the blood vessels in liquid form, was not refreshed so quickly (40% medium was refreshed in the in vitro drug-release model each time). In our opinion, when the drug-loaded substrates were implanted into the bone, the implants were surrounded with bone and hematoma. Therefore, the drug-release kinetics were mainly dependent upon the surrounding hematoma. Other techniques have been reported to prolong drug release from NTs, such as calcium and phosphorus crystal coverage, as well as polymer and vacuum-loading techniques.31–34 Further improvements in regard to filling methods would be required in order to prolong drug release.
Antibacterial ability in vitro
Bacterial adhesion was investigated on Cp-Ti, NT, and NT-V samples. The samples were removed and rinsed with PBS at the end of the prescribed time. The bacteria were stained with Syto-9 and PI for 15 minutes in the dark. Live (green) and dead bacteria (red) were observed by the CLSM. The adhesion and morphology of the bacteria were also investigated by SEM. In addition, the viable bacteria (planktonic bacteria) were investigated in the medium by agar plating. The antibacterial effect on the substrates and the planktonic bacteria in the medium was evaluated, as shown in Figure 3A–D. The results showed that the NT-V effectively reduced bacteria adhesion after 6 hours of culture. No live bacteria grew on the surface of the NT-V or in the culture medium. We found that bacteria morphology was impaired on the surface of NT-V (Figure 3A). The presence of live bacteria in the medium was investigated by agar plating. The medium showed positive bacterial growth in both Cp-Ti and NT groups, with no bacteria growth for the NT-V group. There was a statistically significant reduction in the infection rates in the NT-V group compared to those of the NT and Cp-Ti groups (P<0.01). However, there was no statistically significant difference between the NT and Cp-Ti groups.
Figure 3.
(A–D) Antibacterial efficacy in vitro. (A) The bacteria had a normal morphology on commercially pure titanium (Cp-Ti; white arrow) and TiO2 nanotubes (NTs) (when the NTs were filled with matrix constituents). However, the bacteria morphology was impaired when cultured on the NTs loaded with vancomycin (NT-V; green arrow) and NTs (red arrow), where the NTs were not filled with the matrix constituents. (B) Fluorescence microscopy images of the bacteria stained with Syto–9 and propidium iodide after 2 and 6 hours of culture on Cp-Ti, NT, and NT-V. The live bacteria appeared green while the dead bacteria appeared in red. The confocal laser scanning microscopy images of Staphylococcus aureus colonies after 6 hours of culture on Cp-Ti, NT, and NT-V, respectively. (C) The S. aureus bacterial colonies had a different bacterial density on the tested sample surfaces after 2 and 6 hours. (D) Planktonic bacterial viability in the medium after culture. There were obviously more bacterial colonies on the samples without vancomycin.
Ercan et al25 reported that NTs had a robust antimicrobial effect and that 80 nm-diameter NTs had the most robust antimicrobial effect. In this study, we found that the NTs reduced bacterial adhesion onto the NT surface. Anodization will leave inorganic residue (like [TiF4]2−) on the implant surface. The NTs were sintered at 500°C for 2 hours in our experiments, and the fluorine content was evaporated during sintering.25 Ercan et al25 and Puckett et al35 pointed out that the NTs with high fluorine content on anodized Ti could increase bacterial adhesion on the surface of the anodized Ti. Ercan et al25 further pointed out that heat treatment of NTs (the fluorine content evaporated during sintering) had a tendency to decrease the number of adherent bacteria. As shown in Figure 3C, there was an approximately 40% decrease in bacterial adhesion (both dead and live bacteria) on NTs compared to that of the Cp-Ti after 6 hours. This suggested that the NTs could reduce the S. aureus adhesion. However, we found that the NTs did not kill all the bacteria surrounding the implant (Figure 3A–D). The bacteria could survive on the NT surface, particularly when the NTs were covered with protein (Figure 3A). Figure 3A shows that the bacteria survived on the NT surface; however, the bacterial morphology was impaired on the NT or NT-V surface, as shown by SEM, but not when the surface was covered with matrix constituents. Therefore, the NTs that were covered with different matrix constituents formed a microenvironment, which might have been beneficial for bacterial survival. Ercan et al25 and Puckett et al35 reported that the surface topography, crystalline structure (anatase or rutile), and contact angle of the water influenced antibacterial ability. However, the NTs absorbed the matrix constituents (such as proteins), which could influence their antibacterial ability. Once the NTs were covered with matrix constituents, their antibacterial ability was impaired. This observation regarding bacterial survival on NTs covered with matrix constituents could be useful in anti-infective biomaterial design.
In clinical practice, bacteria could contaminate not only the implants but also the tissues surrounding the implants.36 The bacteria that survived on the NT surface could lead to implant infection; especially when the NT surface was covered with protein. The in vitro study may predict that the NTs cannot prevent implant infection in vivo. Therefore, the in vivo study was designed to investigate the antibacterial efficacy of the NTs and NT-V.
Antibacterial ability in vivo
According to the best of our knowledge, this is the first study to report the antibacterial ability of NTs in an animal model. Figure 2 shows the surgical diagram, where the postoperative X-ray showed correct intramedullary placement of the Ti rods in the femur in all 36 animals (Figure 2C). Thirty days after the implantation, all the animals were killed and histological and microbiological methods were used to detect the infection. In all cases, the postimplant infection was investigated by agar plating and clinical assessment. All animals from the NT-V group were free of infection. Infection rates were 100% (12 of 12 animals) in the Cp-Ti group, and 92% (eleven of 12 animals) in the NT group. There was an excellent correlation between the agar plating (both the rods and the bone sample on the other side) and the clinical findings (Figure 4). In all the infected animals (both the Cp-Ti and NT groups), there was approximately 3 × 105 CFU/mg of bone, which was found in all the serial dilutions. Therefore, there was a statistically significant reduction of the infection rates in the NT-V group as compared to that of the standard Cp-Ti group (P<0.001). The NT-V group had good prophylaxis (no infection). Although the NT group had some robust antibacterial adhesion ability in vitro, it had poor prophylaxis in vivo (Figure 4). The antibacterial ability of the NT-V group toward S. aureus could have been attributed to the initial burst release of the vancomycin from the NT coating.
Figure 4.
(A and B) Antibacterial efficacy in vivo. (A) Microbiological assessment. (B) The animal with the nanotubes (NTs) loaded with vancomycin (NT-V) implants: no culture growth of the Titanium (Ti) rod and bone sample on the agar plates. The animal with TiO2 NT and commercially pure titanium (Cp-Ti) implants: positive culture growth on agar plates of the rolled out Ti rod and the bone sample surrounding the implant.
Poelstra et al reported that a 6-hour postimplant ‘‘decisive period’’ was identified, during which prevention of bacterial adhesion was critical to infection prophylaxis.4 Bacterial adhesion onto the implanted surfaces was critical for the pathogenesis of the implant-related infections.4,5 After the surgery, the bacteria that were not quickly attached onto the implant surface were rapidly destroyed by the immune system.24
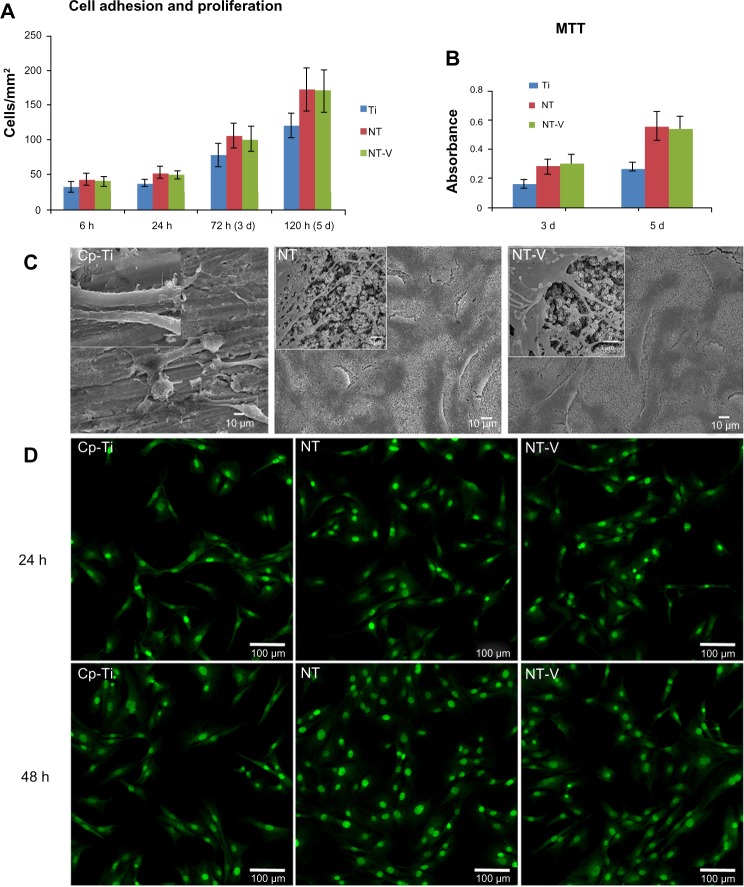
Cell adhesion, proliferation, and morphology
Any new biomaterial must have good biocompatible properties before being used in clinical applications. In this study, the biocompatibility of NT-V was investigated by osteoblast morphology, adhesion, proliferation, and viability on the surface of NTs and NT-V (Figure 5).
Figure 5.
(A–D) Cell behavior on commercially pure titanium (Cp-Ti), TiO2 nanotubes (NTs), and NTs loaded with vancomycin (NT-V). (A and B) Cell adhesion was increased on NTs (both NT and NT-V) compared with the Cp-Ti at 6 and 24 hours (h). At 3 and 5 days (d), the cell numbers had a higher proliferation on the NTs and NT-V compared with the Cp-Ti. (B)The cell proliferation was assessed using MTT-based methods at different time of incubation on the different substrates. (C) Scanning electron microscopy (SEM) micrographs of osteoblasts on Cp-Ti, NT, and NT-V surfaces after 3 days of culture. Higher magnification of the SEM micrographs of osteoblasts on Cp-Ti, NT, and NT-V surfaces showed a much more pronounced protrusion of filopodia, with a significantly longer configuration and a high degree of contact on the NTs (both NT and NT-V) compared to those of the Cp-Ti. The filopodia were also protruding into the nanotube holes on the NTs and NT-V. (D) Fluorescence micrographs of the osteoblast cells after 24 and 48 hours of culture with Cp-Ti, NTs, and NT-V. Living cells (green) and dead cells (red) were stained with acridine orange/ethidium bromide and were visualized using fluorescence microscopy.
As for the adhesion and proliferation studies, osteoblasts were seeded at 1 × 104 cells/cm2. The experiments were conducted under standard cell-culture conditions of 6 and 24 hours’ adhesion and 3 and 5 days of proliferation. Cells adhering onto three random fields (1 mm2 field size) per substrate were counted using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA), and the results were reported as the average cell number (cells/mm2). Figure 5A shows that the nanostructured titanium (both NT and NT-V) improved the adhesion of the osteoblasts as compared to that of the Cp-Ti at 6 and 24 hours. The osteoblast adhesion rate on the NT-V was improved to 34% at 6 hours and to 29% at 24 hours (P<0.05). Figure 5A shows that cell proliferation was improved on NT-V up to 31% on day 3 and up to 42% on day 5 (P<0.05). This suggested that the topographical cues at the nanoscale level promoted cell adhesion and proliferation.
Cell proliferation on the materials (Cp-Ti, NTs, and NT-V) was also determined by the MTT assay. Figure 5B shows the absorbance measured for NT, NT-V, and surfaces after seeding the cells. Cell proliferation was much higher after 3 and 5 days of culturing on NTs and NT-V compared to Cp-Ti (P<0.05). These results were strongly supported by results obtained from several other studies with different types of nanostructured surfaces.15,18,20,21,24 A significant increase (P<0.05) was observed in both NT groups (NT and NT-V) compared to the Cp-Ti group during the entire incubation time, indicating the improved cytocompatibility of the NT coating. There was no significant difference between the NT group and the NT-V group. These results indicated that the NT-V (500 μg/cm2) did not impair nanotubular surface cell-function enhancement.
Cell morphology was also directly affected by the topography of the surface. The shapes of the osteoblasts cultured on Cp-Ti and NTs (both NTs and NT-V) were noticeably different. As observed in the SEM images (after 24 hours of incubation) represented in Figure 5C, the cells spread out on the NTs in a normal manner, forming lamellopodia and wide, thick filopodia, while cell adhesion and spreading was impaired without stable filopodia extension on the smooth TiO2 surface. The highly magnified SEM images were captured to visualize the cell extensions on nanotubular surfaces, with fewer filopodia on the smooth surface. Figure 5C shows a high-magnification SEM image of the osteoblast extension probing into the NTs.
Cell viability
Cell viability was investigated on day 1 and 2 of seeding on NT, NT-V, and Cp-Ti surfaces using the AO/EB assay. The cells were stained with AO/EB at room temperature and were observed on the Olympus CLSM, where the living cells appeared in green and the dead cells appeared in red. Figure 5D shows that all the cells were alive on all the three substrates (Cp-Ti, NT, and NT-V). The results showed that the NT-V group had no negative effects on the osteoblasts. Furthermore, the cells were more viable on nanotubular (both NT and NT-V) surfaces as compared to the smooth surfaces. Oh et al reported that nanotubular titanium promoted osteoblast function, since it initially absorbed greater amounts of proteins from the serum compared to unanodized titanium.15,18,20,37,38 The NTs absorbed more proteins compared to the smooth titanium, which led to improved cell function on the nanotubular surface of the Ti. This difference in cell behavior on the larger-diameter NTs might have been caused by the substantially different nature of the NTs (amorphous, anatase, and rutile phases), as well as different types of cells. Further studies are needed on the effects of NT dimensions, surface chemistry, and crystal structure on the cell-growth behavior of different cell types in order to understand the nature of cell function on NT substrates. In conclusion, these results demonstrated that NT-V (500 μg/cm2) showed not only a good prophylaxis but also good biocompatibility. The dose of vancomycin (500 μg/cm2) in this study did not have any adverse effects on osteoblast function.
This study had several limitations. First, only one type of NT (80 nm in diameter and 800 nm in length) and only one dose of vancomycin (500 μg/cm2) were used in the study. A more detailed evaluation of different types of NTs and different dosages of vancomycin need to be assessed for a more comprehensive study. Second, although the NT-V (500 μg/cm2) group had good in vitro biocompatibility, we did not detect in vivo osseointegration. Further studies should focus on the osseointegration of the NT-V surface in an in vivo study.
Conclusion
NT-V improved antimicrobial activity and biocompatibility both in vitro and in vivo. The dose of vancomycin (500 μg/cm2) in this study did not show adverse effects on the function of the osteoblasts. NTs loaded with antibiotics could be potentially used as implant biomaterial in order to increase the osteoblast function and to decrease implant-related infection rates in orthopedics.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81071449 and 51002027).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Green SA. Complications of external skeletal fixation. Clin Orthop Relat Res. 1983;(180):109–116. [PubMed] [Google Scholar]
- 4.Poelstra KA, Barekzi NA, Rediske AM, Felts AG, Slunt JB, Grainger DW. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res. 2002;60(1):206–215. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2(4):176–194. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 8.Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Titania nanotubes: a novel platform for drug-eluting coatings for medical implants? Small. 2007;3(11):1878–1881. doi: 10.1002/smll.200700412. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 2):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Gulati K, Aw MS, Findlay D, Losic D. Local drug delivery to the bone by drug-releasing implants: perspectives of nano-engineered titania nanotube arrays. Ther Deliv. 2012;3(7):857–873. doi: 10.4155/tde.12.66. [DOI] [PubMed] [Google Scholar]
- 11.van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72(6):557–571. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 12.Berger RA, Jacobs JJ, Quigley LR, Rosenberg AG, Galante JO. Primary cementless acetabular reconstruction in patients younger than 50 years old. 7- to 11-year results. Clin Orthop Relat Res. 1997;(344):216–226. [PubMed] [Google Scholar]
- 13.Albrektsson T, Sennerby L. Direct bone anchorage of oral implants: clinical and experimental considerations of the concept of osseointegration. Int J Prosthodont. 1990;3(1):30–41. [PubMed] [Google Scholar]
- 14.von der Mark K, Park J, Bauer S, Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339(1):131–153. doi: 10.1007/s00441-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 15.Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009;5(8):3215–3223. doi: 10.1016/j.actbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.von Wilmowsky C, Bauer S, Lutz R, et al. In vivo evaluation of anodic TiO2 nanotubes: an experimental study in the pig. J Biomed Mater Res B Appl Biomater. 2009;89(1):165–171. doi: 10.1002/jbm.b.31201. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Li H, Lü W, et al. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials. 2011;32(29):6900–6911. doi: 10.1016/j.biomaterials.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92(3):1218–1224. doi: 10.1002/jbm.a.32463. [DOI] [PubMed] [Google Scholar]
- 19.Eaninwene G, 2nd, Yao C, Webster TJ. Enhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfaces. Int J Nanomedicine. 2008;3(2):257–264. doi: 10.2147/ijn.s2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh S, Daraio C, Chen LH, Pisanic TR, Fiñones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J Biomed Mater Res A. 2006;78(1):97–103. doi: 10.1002/jbm.a.30722. [DOI] [PubMed] [Google Scholar]
- 21.Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28(21):3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Sollazzo V, Pezzetti F, Scarano A, et al. Anatase coating improves implant osseointegration in vivo. J Craniofac Surg. 2007;18(4):806–810. doi: 10.1097/scs.0b013e3180a7728f. [DOI] [PubMed] [Google Scholar]
- 23.Gulati K, Aw MS, Losic D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res Lett. 2011;6:571. doi: 10.1186/1556-276X-6-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popat KC, Eltgroth M, Latempa TJ, Grimes CA, Desai TA. Decreased Staphylococcus Epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28(32):4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Ercan B, Taylor E, Alpaslan E, Webster TJ. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology. 2011;22(29):295102. doi: 10.1088/0957-4484/22/29/295102. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7(6):1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 27.Wei H, Wu S, Feng Z, et al. Increased fibroblast functionality on CNN2-loaded titania nanotubes. Int J Nanomedicine. 2012;7:1091–1100. doi: 10.2147/IJN.S28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arciola CR, Campoccia D, Gamberini S, et al. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials. 2005;26(33):6530–6535. doi: 10.1016/j.biomaterials.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;(333):245–251. [PubMed] [Google Scholar]
- 30.Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29(7):1070–1074. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 31.Moseke C, Hage F, Vorndran E, Gbureck U. TiO2 nanotube arrays deposited on Ti substrate by anodic oxidation and their potential as a long-term drug delivery system for antimicrobial agents. Appl Surf Sci. 2012;258(14):5399–5404. [Google Scholar]
- 32.Gulati K, Ramakrishnan S, Aw MS, Atkins GJ, Findlay DM, Losic D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8(1):449–456. doi: 10.1016/j.actbio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Yao C, Webster TJ. Prolonged antibiotic delivery from anodized nanotubular titanium using a co-precipitation drug loading method. J Biomed Mater Res B Appl Biomater. 2009;91(2):587–595. doi: 10.1002/jbm.b.31433. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Cai K, Luo Z, et al. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012;8(1):439–448. doi: 10.1016/j.actbio.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 36.Hendriks JG, Neut D, van Horn JR, van der Mei HC, Busscher HJ. Bacterial survival in the interfacial gap in gentamicin-loaded acrylic bone cements. J Bone Joint Surg Br. 2005;87(2):272–276. doi: 10.1302/0301-620x.87b2.14781. [DOI] [PubMed] [Google Scholar]
- 37.Oh S, Brammer KS, Li YS, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106(7):2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brammer KS, Oh S, Gallagher JO, Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8(3):786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]