Abstract
Metformin is an oral biguanide commonly used for the treatment of type II diabetes and has recently been demonstrated to possess anti-proliferative properties that can be exploited for the prevention and treatment of a variety of cancers. The mechanisms underlying this effect have not been fully elucidated. Using both in vitro and in vivo models, we examined the effects of metformin on endometrial tumors with defined aberrations in the PI3K/PTEN/mTOR and MAPK signaling pathways to understand metformin mechanism of action and identify clinically useful predictors of response to this agent. In vitro assays of proliferation, cytotoxicity, and apoptosis were used to quantify the effects of metformin on endometrial cancer cell lines with mutations in the PI3K/PTEN/mTOR and MAPK signaling pathways. The in vivo effects of oral metformin on tumor progression were further examined using xenograft mouse models of endometrial cancer. K-Ras localization was analyzed by confocal microscopy using GFP-labeled oncogenic K-Ras and by immunoblot following subcellular fractionation. Metformin inhibited cell proliferation, induced apoptosis, and decreased tumor growth in preclinical endometrial cancer models, with the greatest response observed in cells harboring activating mutations in K-Ras. Furthermore, metformin displaces constitutively active K-Ras from the cell membrane, causing uncoupling of the MAPK signaling pathway. These studies provide a rationale for clinical trials using metformin in combination with PI3K targeted agents for tumors harboring activating K-Ras mutations, and reveal a novel mechanism of action for metformin.
Keywords: metformin, endometrial cancer, K-Ras, PTEN
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States. An estimated 47,130 women were diagnosed with uterine cancer in 2012, and an estimated 8,010 of these women will die of the disease (1). Type I ECs account for approximately 80% of cases, are classically of endometrioid histology, and are associated with obesity in up to 90% of cases. Additionally, type II diabetes is associated with an increased risk for the development of EC (2, 3).
The oral biguanide, metformin, is one of the most commonly used hypoglycemic agents in the management of type II diabetes. Epidemiological studies have demonstrated that diabetic patients being treated with metformin have a reduced cancer incidence or improved response to chemotherapy when compared to patients receiving other oral hypoglycemic agents or insulin (4-6). A meta-analysis of five observational studies of all cancer types found that metformin was associated with a 31% decrease in cancer risk (6). A study of 2,529 patients who received neoadjuvant chemotherapy for early-stage breast cancer, showed that the rate of pathologic complete responses was 24% among diabetic patients using metformin versus 8% for diabetic patients not using metformin (p<0.007) (4). Rodent models have provided additional support for the cancer preventive effects of metformin (7-9).
The mechanisms through which metformin decreases circulating glucose and insulin levels are well known, but recent studies have suggested that metformin also has direct anti-cancer effects. In fact, the direct effects of metformin are hypothesized to parallel mechanisms involved in the management of diabetes. Specifically, metformin inhibits mitochondrial adenosine-5′-triphosphate (ATP) production, resulting in activation of the cellular energy-sensing LKB1/AMPK pathway (10-12). (LKB1 is also known as serine/threonine kinase 11, STK11). AMPK activation inhibits cellular proliferation and mRNA translation via mammalian target of rapamycin (mTOR) signaling, which may contribute to the direct anti-neoplastic effects of metformin. Interestingly, anti-tumor and cancer preventive effects were reported for other biguanides decades ago (13-16), but little mechanistic follow-up has been conducted.
Aberrations in mTOR signaling have been implicated in the development of EC. The most frequent genetic aberration seen in Type I ECs is loss of function of the tumor suppressor phosphatase and tensin homolog (PTEN), which occurs in up to 83% of lesions and is thought to be an early event in tumorigenesis (17). PTEN most notably plays a role in the regulation of the PI3K/AKT/mTOR pathway by inhibiting the downstream phosphorylation of AKT. Loss of functional PTEN results in increased mTOR signaling and promotes cellular proliferation. Given the prevalence of PI3K/AKT/mTOR pathway alterations associated with EC and its frequent association with obesity and diabetes, EC is an ideal site in which to evaluate the role of metformin as a cancer therapeutic. However, to date, studies on the effects of metformin on EC are limited.
In addition to alterations in PTEN, K-Ras mutations represent another common genetic defect found in endometrial cancers. Activating mutations in K-Ras are observed in 15-26% of endometrioid carcinomas (18, 19) and have been shown in a mouse model to synergize with PTEN inactivation to accelerate tumorigenesis in endometrial and lung cancer (20, 21). Crosstalk between PI3K/AKT and K-Ras signaling pathways is clinically significant, particularly in endometrial cancer where these pathways are both frequently mutated, because it suggests the need to target more than one pathway to inhibit cancer cell proliferation and tumor growth. For this reason, we sought to characterize the effects of metformin on endometrial tumors in which K-Ras is activated, either in isolation or accompanied by loss of PTEN.
The identification of differential responses to metformin based on the genetic fingerprint of individual endometrial tumors may have important implications in defining a subset of patients that will benefit from metformin therapy either alone or in combination with other targeted agents. Furthermore, an understanding of molecular mechanisms underlying metformin’s anti-proliferative effect may lead to the development of novel combination therapies for more effective treatment of a variety of cancers.
Methods
Cell Culture and Reagents
Hec1A, a well-differentiated human endometrial carcinoma cell line that expresses PTEN and harbors an activating K-Ras mutation (K-RasG12D) was purchased in 2012 from the American Type Culture Collection (ATCC, Manassas, VA). Ishikawa, a well-differentiated human endometrial carcinoma cell line with loss of PTEN expression and wild-type K-Ras was purchased from the European Collection of Cell Cultures in 2012 (ECACC, Porton Down, United Kingdom). MecPK, a mouse endometrial carcinoma cell line were derived in our laboratory in 2012 from the endometrial tumor of a transgenic mouse with homozygous PTEN deletion and an activating K-Ras mutation (PTEN−/−K-RasG12D) (21) and were validated for both K-Ras mutation and PTEN status.. All endometrial cancer cell lines included in this study express OCT1, an transporter required for metformin uptake and response (22). Original stock of Madin-Darby Canine Kidney (MDCK) cells were purchased from ATCC in 2007. All experiments were conducted with low passage cells from recently resuscitated frozen stocks. Cell lines obtained from ATCC and ECACC were subjected to comprehensive quality control and authentication procedures, including short tandem repeat profiling for human cell lines, species verification by DNA barcoding, verification of morphology, and testing for fungi, mycoplasma, and other bacteria. Metformin (1,1-Dimethylbiguanide hydrochloride) was purchased from Sigma (St. Louis, MO). Aminoimidazole-4-Carboxamide Riboside (AICAR) was obtained from Cell Signaling (Beverly, MA).
Metformin Treatment Cell Proliferation Assays
EC cells were seeded in 96-well plates at a density of 4 × 103 cells/well 24 hours prior to drug treatment. The medium was then replaced with antibiotic-free medium containing metformin. After treatment for 48 hours, cells were incubated for 3.5 hours with MTT (3-(5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye at 37°C under 5% CO2. Following the incubation, the MTT reaction was halted with the addition of solvent containing 2-propanol, 0.1% NP40, and 4 mM HCL. The absorbance was measured at a wavelength of 590nm with 620nm as a background. Three independent assays were performed in triplicate.
Western Blot
EC cells were treated for 48 hours and whole cell lysates were collected with Mammalian Protein Extraction Reagent (ThermoScientific, Rockford, IL). Lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane, which was incubated overnight at 4°C with 1:1000-2000 dilution of primary antibody. Antibodies against phospho-AMPKα (Thr172), total AMPK, phospho-Akt (Ser473), total Akt, phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), phospho-S6 ribosomal protein (Ser235/236), and PTEN were purchased from Cell Signaling. Antibodies against β-actin and organic cation transporter-1 (OCT1) were purchased from Sigma. Incubation with horseradish peroxidase-conjugated secondary antibody was performed for 1 hour. SuperSignal® West Dura Extended Duration Substrate (ThermoScientific) was used to enhance signal.
Cell Cycle Analysis
After treatment, cells were fixed in 70% ethanol for a minimum of 24 hours at 4°C. Cells were stained at room temperature for 30 minutes using a solution of 40 μg/mL propidium iodide, 80 μg/mL RNase A in PBS supplemented with 0.1% Triton X-100 and 0.1 mM EDTA. Cells were incubated in staining solution at room temperature for 30 minutes before cell cycle analysis was performed using the Gallios Flow Cytometer (Beckman Coulter, Indianapolis, IN) according to manufacturer’s protocol. Kaluza Flow Cytometry Analysis v1.1 software (Beckman Coulter) was used to calculate cell cycle distribution. Apoptotic cells were determined as the percentage of cells in the sub-G1 peak.
Animal Studies
Female nude mice (athymic NCr-nu/nu) were purchased from the National Institutes of Health (Bethesda, MD) and maintained in accordance with Institutional Animal Care and Use Committee guidelines. At 6 weeks of age, intraperitoneal injections were performed with 5 × 106 early-passage endometrial cancer cells (20 mice injected with Hec1A cells, 20 injected with Ishikawa cells, and 20 injected with MecPK cells). Tumors were allowed to progress for 7 days prior to initiating either control or metformin treatment. Each group of 20 mice per cell line was divided into two subgroups of 10 mice each. Ten mice were treated with metformin dissolved in drinking water (5 mg/mL) and the remaining ten mice were provided untreated sterile drinking water. Both metformin-treated water and control untreated water were changed twice weekly. Treatment continued until at least one mouse became moribund, at which time all animals in the same endometrial cancer cell line group were euthanized. At necropsy, mice were weighed and serum was collected for liver enzyme analysis. All tumor was dissected from peritoneal surfaces and weighed, a representative portion of tumor was fixed in formalin, and the remainder was flash frozen and stored at −80°C.
Immunohistochemistry
Slides were cut from formalin-fixed, paraffin-embedded tissue blocks for immunohistochemical analysis of phosphorylated S6 ribosomal protein (pS6rp) expression as a downstream marker of PI3K/AKT pathway signaling in tumor xenografts. Incubation with primary antibody for phosphorylated S6 ribosomal protein (S235/236) rabbit monoclonal antibody (Cell Signaling Technology) was performed overnight at 4°C (1:50 dilution). Phosphorylated S6rp expression was scored as the product of the percentage of cells staining positive (0 = <10%; 1 = 10-25%; 2 = 26-50%, 3 = 51-75%, and 4 = >75%) and the intensity of the staining (1 = weak; 2 = moderate; 3 = strong).
Stable Expression of PTEN
MecPK cells were transfected with either pIRES2-EGFP-PTEN, pIRES2-EGFP control (Clontech Laboratories, Mountain View, CA) or Lipofectamine 2000 alone (mock transfection). After 24 hours, G418-sulfate 750 ug/mL (Cellgro, Manassas, VA) was applied and replaced every 3 days until resistant colonies were identified. Colonies were selected and expanded. Whole cell lysates were analyzed for PTEN expression by western blot. MecPK cells stably expressing PTEN and negative controls transfected with pIRES2-EGFP vector alone were treated with metformin and MTT assays were performed 48 hours later.
Silencing of Mutant K-Ras
MecPK cells were transfected with either siGENOME SMARTpool K-Ras siRNA or siGENOME non–targeting siRNA (Dharmacon, Lafayette, CO) using RNAiMax (Invitrogen, Carlsbad, CA) per manufacturer’s protocol. Media was replaced after 6 hours with media containing metformin. After treatment for 48 hours, MTT assays were performed.
Imaging Analysis of K-Ras Localization
K-Ras localization was analyzed by confocal microscopy using Madin-Darby Canine Kidney (MDCK) cells expressing GFP-labeled oncogenic K-RasG12V or GFP-K-RasG12V/S181A (a mutant K-Ras protein that is resistant to Protein Kinase C (PKC)-dependent phosphorylation). MDCK cells were cultured on coverslips and treated with PBS or metformin for 48 hours to allow new protein synthesis and trafficking. Cells were fixed with 4% paraformaldehyde at room temperature for 30 minutes and quenched with 50mM ammonium chloride for 10 minutes. Coverslips were mounted onto slides in Mowiol and imaged by confocal microscopy using a Nikon A1R Confocal Laser Microscope (Nikon, Tokyo, Japan). Three independent experiments were performed in which three representative fields from each slide were imaged and analyzed using ImageJ software. The ratio of membrane-bound GFP-labeled K-Ras to total (membrane + cytoplasmic) K-Ras was calculated.
Subcellular Fractionation
Hec1A cells were treated with PBS, metformin, or Aminoimidazole-4-Carboxamide Riboside (AICAR, an AMP analog that promotes activation of AMPK by increasing the ratio of intracellular AMP to ATP) for 48 hours and then subjected to subcellular fractionation (Subcellular Protein Fractionation Kit, ThermoScientific). Protein was separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Rabbit monoclonal Na+/K+ ATPase alpha antibody (Epitomics, Burlingame, CA) and total AKT (Cell Signaling) were used as loading controls and to confirm purity of membrane and cytoplasmic fractions, respectively.
Western blot was performed using anti-c-K-Ras, clone 234-4.2 (Sigma). Densitometry was analyzed using ImageJ software and ratio of membrane-bound K-Ras to total (membrane + cytoplasmic) K-Ras was calculated. Three independent experiments were performed in triplicate.
Statistical Analyses
The values presented are the mean ± SEM and analyzed using the Student’s t-test or the Mann-Whitney U test. IC50 values were calculated using GraphPad Prism. For all results, significance was set as p < 0.05.
Results
Metformin Inhibits Proliferation in Cells Harboring K-Ras Mutation
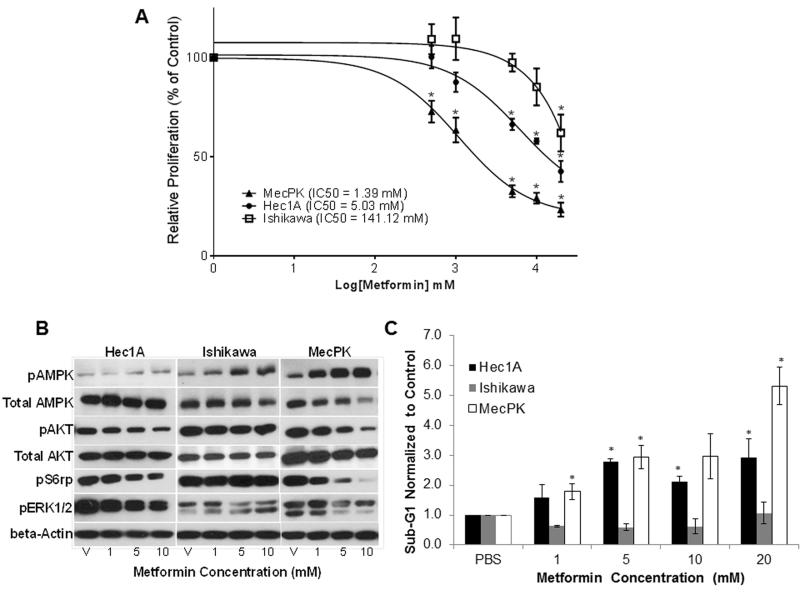
Metformin significantly inhibited proliferation of MecPK (PTEN-null with activating K-Ras mutation) and Hec1A (PTEN wild-type with activating K-Ras mutation) cells in a concentration-dependent manner (Figure 1A). A significant decrease in cell proliferation was achieved at 0.5 mM (p=0.008) and 5 mM (p<0.001), respectively. The mean IC50 was 1.39 mM for MecPK and 5.03 mM for Hec1A. In contrast, proliferation of Ishikawa cells (PTEN-null with wild-type K-Ras) was significantly inhibited only at 20 mM (p=0.015) and the IC50 was 141.12 mM.
Figure 1.
A) Dose-response curves for cell proliferation following metformin treatment in Hec1A, Ishikawa, and MecPK endometrial cancer cell lines. Cells were treated with metformin for 48 hours and cell proliferation was determined using MTT assay. Values were normalized to PBS vehicle (V) controls. Error bars indicate +/− SEM. * p<0.05. B) Western immunoblots for protein expression following metformin treatment after 48h. C) Effect of metformin on apoptosis (proportion of cells in the sub-G1 phase). Cells were treated with metformin or vehicle (PBS) for 48 hours and cell cycle was analyzed using flow cytometry. Apoptotic cells were determined on the histogram as the percentage of cells in the sub-G1 peak. Data represent three independent experiments. Error bars indicate +/− SEM. *p<0.05 indicating increased apoptosis.
Metformin Effects on Cell Signaling
Effects on AMPK activation and downstream mTOR and MAPK pathways were evaluated by treating EC cells with metformin (1–10 mM) for 48 hours (Figure 1B). Metformin induced concentration-dependent phosphorylation of AMPK in all cell lines. Metformin treatment altered downstream mTOR signaling as shown by a concentration-dependent decrease in phosphorylation of S6 ribosomal protein (S6rp) in Hec1A and MecPK cells. However, metformin did not alter S6rp phosphorylation in Ishikawa cells. Extracellular-signal-regulated kinase 1 and 2 (ERK1/2) are downstream targets of the MAPK pathway. Similar to the effect seen on S6rp, metformin induced a concentration-dependent decrease in phosphorylation of ERK1/2 in Hec1A and MecPK cells, but not in Ishikawa cells, suggesting that metformin may alter MAPK signaling. Although AKT is upstream of TSC-2 (the direct target of AMPK), metformin induced a decrease in phosphorylation of AKT in both Hec1A and MecPK cells, suggesting that metformin may have effects upstream in the PI3K pathway independent of effects on AMPK. This effect was not seen in Ishikawa cells. Immunoblotting confirmed that Hec1A cells expressed PTEN while Ishikawa and MecPK cells did not. All cell lines expressed OCT-1, the transporter that is essential for metformin entry into cells (22) (Supplementary Figure S1).
Metformin Induces Apoptosis in K-Ras Mutated Cells
Metformin is a potent inducer of apoptosis in Hec1A and MecPK cells with significant increases in the fraction of apoptotic cells seen at concentrations of 5 mM (p<0.001) and 1 mM (p=0.048), respectively (Figure 1C). However, metformin did not induce apoptosis in Ishikawa cells.
Metformin Preferentially Inhibits Tumor Growth in Cells with K-Ras Mutations
Athymic nude mice received metformin in drinking water at an average daily dose of approximately 1 g/kg/day. Water intake was not altered due to addition of metformin in drinking water (data not shown). The time to development of moribund symptoms following intraperitoneal tumor cell injection was 50 days for the Hec1A group, 64 days for the Ishikawa group, and 29 days for the MecPK group. All 20 (100%) mice injected with Hec1A, all 20 (100%) mice injected with Ishikawa, and 17 of 20 mice (85%) injected with MecPK developed tumors. As shown in Table 1, metformin caused a significant decrease in mean tumor weight compared to untreated mice (0.22 g vs. 0.40 g, p=0.002) in the Hec1A group and MecPK group (0.72 g vs. 1.37 g, p=0.024). In contrast, there was no difference in tumor weights between metformin-treated and untreated mice (0.87 g vs. 1.12 g, p=0.337) in the Ishikawa group. Immunohistochemical staining for pS6rp was performed to confirm modulation of the PI3K/AKT pathway in tumor xenografts. Consistent with in vitro results, expression of pS6rp was significantly decreased in metformin-treated Hec1A tumors compared to vehicle-treated mice (mean IHC score 4.9 vs. 7.8, p=0.009) – (Supplementary Figure S2). In contrast, there was no significant difference in the expression of pS6rp in metformin-treated Ishikawa tumor tissue compared to control. MecPK xenografts showed substantial infiltration by normal cells, making IHC scoring difficult (data not shown).
Table 1.
Effect of Metformin on Tumor Weight in Mouse Xenografts
| Cell Line |
Mice (n) |
Developed Tumor n (%) |
Time to Moribund (days) |
Mean Tumor weight (g) |
Mean Mouse Weight (g) |
Mean Serum ALTa (U/L) |
|---|---|---|---|---|---|---|
| Hec1A | 20 | 20 (100%) | 50 | Control: 0.40 Metformin: 0.22 *p=0.002 |
Control: 27.01 Metformin: 25.97 p=0.281 |
Control: 17.5 Metformin: 22.0 p=0.228 |
| Ishikawa | 20 | 20 (100%) | 64 | Control: 1.12 Metformin: 0.87 p=0.337 |
Control: 26.28 Metformin: 26.24 p=0.971 |
Control: 30.0 Metformin: 37.7 p=0.616 |
| MecPK | 20 | 17 (85%) | 29 | Control: 1.37 Metformin: 0.72 *p=0.024 |
Control: 26.41 Metformin: 24.95 p=0.420 |
Control: 13.7 Metformin: 17.0 p=0.059 |
Alanine aminotransferase (ALT), a marker of liver toxicity.
As markers for toxicity, we compared total body weight at the time of necropsy and serum liver enzyme levels. Mean mouse weights were not significantly different between groups (Table 1). Also, metformin treatment did not adversely affect serum liver ALT enzyme levels (Table 1).
PTEN Status Does Not Alter the Effect of Metformin on Proliferation
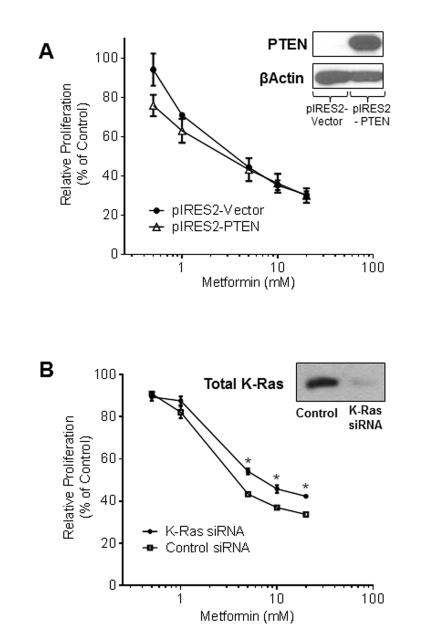
In order to confirm the influence of individual mutations on metformin response, MecPK cells were transfected with pIRES2-PTEN, resulting in stable expression of PTEN. However, expression of PTEN did not alter response to metformin (as measured by changes in relative cell proliferation) compared to control MecPK cells, which are PTEN null (Figure 2A). The mean IC50 for control MecPK cells was 3.31 mM and for MecPK cells expressing PTEN it was 3.0 mM.
Figure 2.
A) MecPK cells were transfected for stable expression of PTEN. PTEN-expressing MecPK cells were treated with metformin for 48 hours and cell proliferation was measured and normalized to PBS controls. B) Mutant K-Ras was transiently silenced in MecPK cells using siRNA. MecPK cells with transiently silenced K-Ras were treated with metformin for 48 hours, resulting in decreased sensitivity to metformin compared to MecPK cells treated with non-target siRNA (negative control). Error bars indicate +/− SEM.
Mutant K-Ras Silencing Decreases the Effect of Metformin on Cell Proliferation
To further confirm the influence of individual mutations on metformin response, transient silencing of K-Ras was performed in MecPK cells using targeted siRNA to decrease expression of total K-Ras (Figure 2B). In both groups, metformin decreased relative cell proliferation compared to controls (Figure 2B), indicating that K-Ras silencing alone is not sufficient to completely abrogate metformin’s effect on proliferation. However, cells with silenced K-Ras demonstrated decreased sensitivity to metformin treatment compared to MecPK cells transfected with non-targeted siRNA (Figure 2B), confirming the importance of K-Ras status for metformin response in MecPK cells.
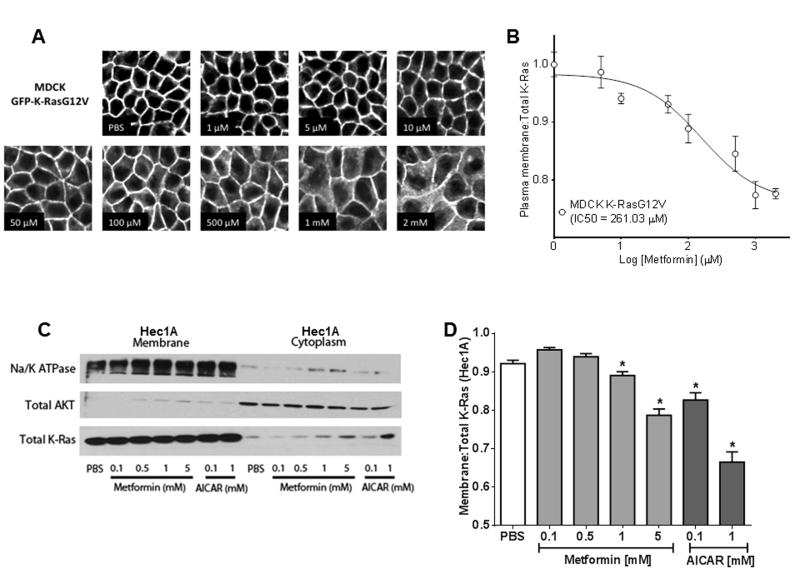
Metformin Induces Mislocalization of K-Ras to the Cytoplasm
K-Ras signaling is silenced if K-Ras is displaced from the plasma membrane. To explore this possible mechanism for metformin action, we used MDCK cells expressing GFP-K RasG12V (a model for quantitatively analyzing K-Ras localization) to evaluate metformin-induced changes to K-Ras localization. Metformin caused a concentration-dependent translocation of K-Ras from the plasma membrane to endomembranes and the cytoplasm (Figure 3A), yielding an IC50 of 261.03 μM (Figure 3B). Imaging analysis of K-Ras localization was not feasible in Hec1A or MecPK cell lines because of a tendency to grow in multiple layers when confluent, in contrast to the MDCK cells, which grow in a confluent monolayer allowing for clear image analysis. For this reason, the effect of metformin on K-Ras localization was further examined in EC cells using subcellular fractionation and immunoblot. In Hec1A cells, metformin treatment caused a small, but significant, concentration-dependent mislocalization of K-Ras from the membrane fraction to the cytosolic fraction at 1 and 5 mM of metformin (p=0.006 and p<0.001) (Figure 3C and D). Taken together with the more extensive K-Ras mislocalization evident in the imaging assay this result suggests that K-Ras displaced from the plasma membrane by metformin treatment is predominantly associated with endomembranes. Importantly, treatment with AICAR (an AMP analog that promotes activation of AMPK) also resulted in a concentration-dependent mislocalization of K-Ras from the membrane to the cytosolic fraction at 0.1 mM (p=0.0002) and 1 mM (p<0.0001) (Figure 3C and D).
Figure 3.
Metformin induces mislocalization of K-Ras. A) K-Ras localization following metformin treatment analyzed by confocal microscopy in Madin-Darby Canine Kidney (MDCK) cells expressing GFP-labeled oncogenic K-RasG12V mutant. B) Quantitative image analysis was performed to determine ratio of K-Ras at the plasma membrane versus total K-Ras (normalized to PBS-treated controls). Representative experiments shown, three independent experiments were performed. C) Immunoblot of Hec1A subcellular fractionation following 48 hours of metformin or AICAR treatment. D) Quantitation of fractionation immunoblot shows a concentration-dependent translocation of K-Ras from the membrane to the cytoplasm compared to PBS treatment. Error bars indicate +/− SEM. *p<0.05
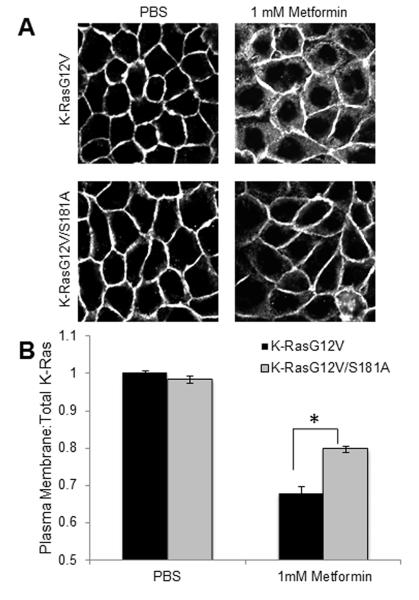
Metformin Induces Mislocalization of K-Ras via Protein Kinase C (PKC)
To further probe the mechanism of metformin-induced K-Ras mislocalization, we used MDCK cells expressing GFP-K-RasG12V/S181A, a mutant protein that is resistant to PKC-dependent phosphorylation. MDCK cells expressing the resistant mutant K-RasS181A showed significantly less K-Ras mislocalization following metformin treatment compared to MDCK cells expressing K-Ras that is sensitive to PKC-dependent phosphorylation (Figure 4), suggesting that metformin acts on K-Ras localization through a PKC-dependent mechanism.
Figure 4.
Mislocalization of K-Ras occurs via Protein Kinase C. A) K-Ras localization following metformin treatment analyzed by confocal microscopy in Madin-Darby Canine Kidney (MDCK) cells expressing GFP-labeled oncogenic K-RasG12V or the mutant K-RasG12V/S181A that is resistant to Protein Kinase C (PKC)-dependent phosphorylation. B) Quantitative image analysis of the ratio of K-Ras at the plasma membrane versus total K-Ras shows that metformin-induced K-Ras mislocalization is blunted in the PKC-resistant mutant. Representative images shown, three independent experiments were performed. Error bars indicate +/− SEM. *p<0.05
Discussion
The chemopreventive and antineoplastic effects of metformin are currently being evaluated for the treatment of several cancers. Although its mechanism of action is not fully understood, metformin is thought to inhibit cell proliferation locally via activation of the AMPK signaling pathway, which counteracts the growth-promoting effects of the PI3K/AKT/mTOR pathway. PI3K pathway activation, due to inactivating mutation of the PTEN tumor suppressor gene, is commonly observed in type I endometrial cancer. In our study, metformin caused concentration-dependent activation of AMPK in all three endometrial cancer cell lines tested; however, differential effects on other pathways were dependent on mutational status. Our in vitro findings show that endometrial cancer cells expressing constitutively active K-Ras are more responsive to metformin (as shown by downregulation of AKT and ERK signaling and increased apoptosis) compared to cells that lack PTEN. A xenograft mouse model provided in vivo confirmation, showing growth of Hec1A and MecPK tumors was inhibited by metformin therapy, while Ishikawa tumors were resistant. This demonstrates that mutational status of K-Ras may be more predictive of tumor response to metformin than PTEN status.
While mutations in the K-Ras and PI3K pathway components are mutually exclusive in some tumor types, they frequently coexist in endometrial and colon cancer (23-26). However, our results strongly suggest that metformin is a more potent inhibitor of cellular proliferation and in vivo tumor growth in endometrial cancer cells expressing activated K-Ras regardless of PTEN expression status. We next investigated the molecular basis for the increased toxicity of metformin against endometrial cancer cells expressing activated K-Ras. K-Ras undergoes a series of post-translational modifications of the C-terminal CAAX motif to generate a farnesyl-cysteine carboxymethyl ester lipid anchor that operates in conjunction with an adjacent polylysine domain to attach K-Ras to the inner leaflet of the plasma membrane (27). Correct localization to the plasma membrane is essential for K-Ras biological activity, since Ras effectors must be recruited to the plasma membrane for activation (28). Our imaging and cell fractionation studies provide compelling evidence that metformin and AICAR displace oncogenic mutant K-Ras from the plasma membrane to cytosol and endomembranes. This change in sub-cellular localization can fully account for the abrogation of K-Ras dependent MAPK activation observed in metformin-treated cells. K-Ras has been shown to translocate to endomembranes due to phosphorylation of a critical serine residue (S181) in the membrane-anchoring polybasic domain. Phosphorylation reduces the positive charge of the K-Ras polybasic basic domain compromising electrostatic interactions with the negatively charged plasma membrane, the so-called “farnesyl-electrostatic switch” (29). Phosphorylation in turn promotes the association between oncogenic K-Ras and Bcl-XL on the mitochondrial outer membrane, inducing apoptosis (29). In this context, recent studies have shown that metformin and AICAR potently activate atypical PKCζ (30, 31). Combined with our findings, this suggests that PKCζ-mediated phosphorylation of K-Ras is the likely mechanism by which metformin uncouples K-Ras signaling from membrane bound growth factor receptors and promotes apoptosis of tumor cells (Figure 5). Earlier work implicated ionophore-sensitive “typical” PKC isoforms in operating the K-Ras farnesyl-electrostatic switch, our new data here now suggest that the switch can also be triggered by atypical PKC. Other drugs that have been shown to displace K-Ras from the plasma membrane include: chlorpromazine, a weak amphiphilic base that neutralizes the inner plasma membrane negative ζ–potential to interfere with K-Ras electrostatic interactions (32); fendiline, via an as yet uncharacterized mechanism (33); and staurosporines that deplete the inner leaflet of phosphatidylserine (34). These drugs, however, are equally active on K-RasS181A, a mutant that cannot undergo PKC-dependent phosphorylation, whereas K-RasS181A was resistant to mislocalization by metformin, providing further evidence for the PKC-dependent mechanism of action depicted in Figure 5.
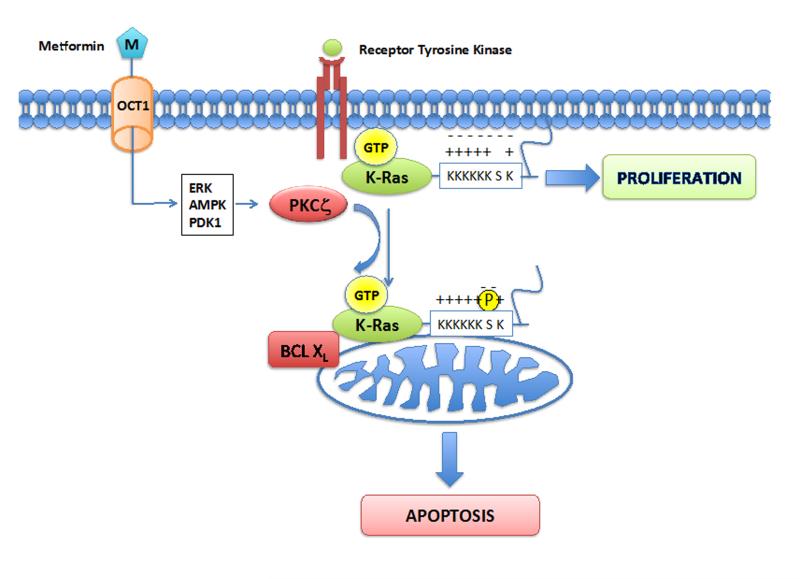
Figure 5.
Proposed mechanism by which metformin causes translocation of activated K-Ras from the plasma membrane and promotes cell death. Previous studies have shown that metformin activates atypical PKC (PKCζ) through an AMPK-, ERK-, and PDK1-dependent mechanism. PKCζ then phosphorylates the lysine rich tail of activated K-Ras, acting as an electrostatic switch that causes K-Ras to be repelled from the membrane. This phosphorylation also promotes association between oncogenic K-Ras and Bcl-XL on the mitochondrial outer membrane, inducing apoptosis.
Our observations have important clinical implications for endometrial cancer treatment, particularly endometrioid-type, as up to 26% harbor activating K-Ras mutations and up to 83% have PTEN loss with subsequent PI3K pathway hyperactivation. Several targeted strategies have been evaluated for treatment of endometrial carcinoma (35). Unfortunately, some combination therapies involving PI3K pathway inhibitors with MEK inhibitors have proven to be toxic in Phase I studies making their development into feasible treatment strategies uncertain. Using metformin to target the Ras-MAPK signaling pathway may be useful when combined with other agents targeting the PI3K pathway and may reduce toxicity. Furthermore, our observations provide a rationale for use of metformin in other cancer types with high incidence of K-Ras mutations, such as pancreatic and colorectal cancer. Indeed, retrospective studies show metformin to be associated with a survival advantage in patients with pancreatic and colorectal cancer (36, 37). Prospective studies are needed to validate these findings.
In conclusion, metformin significantly inhibits cell proliferation, induces apoptosis, and decreases tumor growth in preclinical models of endometrial cancer. Metformin is most effective against endometrial tumors with activating mutations in K-Ras. Metformin inhibits K-Ras signaling through mislocalization of K-Ras from the plasma membrane to the cytoplasm. Metformin’s effects on K-Ras may provide added benefit when combined with other targeted agents, notably mTOR inhibitors, to improve responses. These studies reveal a novel mechanism of action for metformin, and provide a rationale for clinical trials using metformin in combination with PI3K-targeted agents for tumors harboring activating K-Ras mutations.
Supplementary Material
Acknowledgments
Financial support: National Institutes of Health grant P50CA098258 to K.H. Lu and R.R. Broaddus, the National Research Service Award T32 CA101642 for D.A. Iglesias, MD Anderson’s Cancer Center Support Grant CA016672 and Cancer Prevention & Research Institute of Texas grant RP100483 to J.F. Hancock.
Abbreviations
- AICAR
Aminoimidazole-4-Carboxamide Riboside
- EC
endometrial cancer
- MDCK
Madin-Darby Canine Kidney cells
- mTOR
mammalian target of rapamycin
- MTT
3-(5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OCT1
organic cation transporter-1
- pS6rp
phosphorylated S6 ribosomal protein
- PKC
Protein Kinase C
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Pandey A, Forte V, Abdallah M, Alickaj A, Mahmud S, Asad S, et al. Diabetes mellitus and the risk of cancer. Minerva endocrinologica. 2011;36:187–209. [PubMed] [Google Scholar]
- 3.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. American journal of obstetrics and gynecology. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 7.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocrine-related cancer. 2008;15:833–9. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 8.Bojkova B, Orendas P, Garajova M, Kassayova M, Kutna V, Ahlersova E, et al. Metformin in chemically-induced mammary carcinogenesis in rats. Neoplasma. 2009;56:269–74. doi: 10.4149/neo_2009_03_269. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 10.Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Cantley LC. Chemoprevention meets glucose control. Cancer Prev Res (Phila) 2010;3:1049–52. doi: 10.1158/1940-6207.CAPR-10-0178. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 13.Byczkowski JZ, Zychlinski L, Porter CW. Potentiation of the antimitochondrial and antiproliferative effects of bis(guanylhydrazones) by phenethylbiguanide. Cancer Res. 1982;42:3592–5. [PubMed] [Google Scholar]
- 14.Dilman VM, Anisimov VN. Potentiation of antitumor effect of cyclophosphamide and hydrazine sulfate by treatment with the antidiabetic agent, 1-phenylethylbiguanide (phenformin) Cancer letters. 1979;7:357–61. doi: 10.1016/s0304-3835(79)80066-x. [DOI] [PubMed] [Google Scholar]
- 15.Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–6. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 16.Dilman VM, Berstein LM, Zabezhinski MA, Alexandrov VA, Bobrov JF, Pliss GB. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Archiv fur Geschwulstforschung. 1978;48:1–8. [PubMed] [Google Scholar]
- 17.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 18.Okuda T, Sekizawa A, Purwosunu Y, Nagatsuka M, Morioka M, Hayashi M, et al. Genetics of endometrial cancers. Obstet Gynecol Int. 2010;2010:984013. doi: 10.1155/2010/984013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–24. [PubMed] [Google Scholar]
- 20.Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–27. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, et al. The synergistic effect of conditional Pten loss and oncogenic K-Ras mutation on endometrial cancer development occurs via decreased progesterone receptor action. J Oncol. 2010;2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal ED, Yasmeen A, Beauchamp MC, Rosenblatt J, Pollak M, Gotlieb WH. Relevance of the OCT1 transporter to the antineoplastic effect of biguanides. Biochem Biophys Res Commun. 2011;414:694–9. doi: 10.1016/j.bbrc.2011.09.134. [DOI] [PubMed] [Google Scholar]
- 23.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer. 2011;2:261–74. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–73. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 25.Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, et al. PIK3CA cooperates with other phosphatidylinositol 3′-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 2008;68:8127–36. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 26.Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Jr., et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649–54. doi: 10.1016/j.ejca.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Biol. 2012;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prior IA, Hancock JF. Ras trafficking, localization and compartmentalized signalling. Semin Cell Dev Biol. 2012;23:145–53. doi: 10.1016/j.semcdb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–93. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Nimal S, Longnus SL, et al. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab. 2010;298:E179–92. doi: 10.1152/ajpendo.00392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberg S, Giehl K, Henis YI, Ehrlich M. Differential interference of chlorpromazine with the membrane interactions of oncogenic K-Ras and its effects on cell growth. Journal of Biological Chemistry. 2008;283:27279–88. doi: 10.1074/jbc.M804589200. [DOI] [PubMed] [Google Scholar]
- 33.van der Hoeven D, Cho KJ, Ma X, Chigurupati S, Parton RG, Hancock JF. Fendiline inhibits k-ras plasma membrane localization and blocks k-ras signal transmission. Mol Cell Biol. 2013;33:237–51. doi: 10.1128/MCB.00884-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho KJ, Park JH, Piggott AM, Salim AA, Gorfe AA, Parton RG, et al. Staurosporines disrupt phosphatidylserine trafficking and mislocalize ras proteins. J Biol Chem. 2012;287:43573–84. doi: 10.1074/jbc.M112.424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–71. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 36.Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374–8. doi: 10.1038/bjc.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–12. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.