Figure 1. Schematic overview of LINC complex proteins and their connections to the cytoskeleton and nuclear interior.
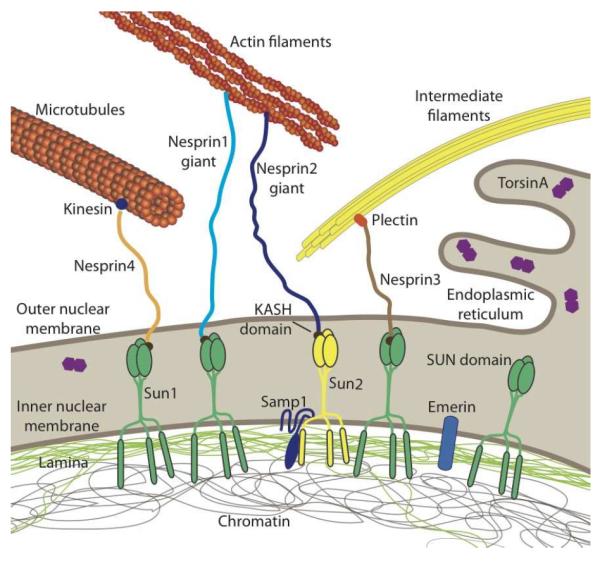
SUN proteins at the inner nuclear membrane bind to the nuclear lamina and other nucleoplasmic proteins while interacting with KASH-domain containing proteins at the outer nuclear membrane. KASH-domain containing proteins directly or indirectly interact with cytoskeletal filaments, thereby forming a physical connection between the nuclear interior and cytoskeleton. Please note that SUN- and KASH domain proteins can exist in multiple isoforms encoded by several genes. In human somatic cells, the most predominant KASH-domain proteins are nesprin-1, -2, and -3 and their various isoforms, and Sun1 and Sun2 as the predominant SUN proteins [16]. Illustrated are only the largest isoforms for nesprins1–4; cells express many additional shorter nesprin isoforms, including some lacking the KASH domain. Smaller nesprin isoform may also be located on the inner nuclear membrane. Note that nesprin-1, -2, -4 and KASH5 can also interact with kinesin and/or dynein. Samp1 and torsinA are involved in the regulation of the LINC complex. Not depicted are KASH5 and the SUN protein isoforms Sun3–5, as their expression is restricted to germ cells. The nuclear lamina comprises A-type and B-type lamins. Note that torsinA can be localized in the endoplasmic reticulum and the perinuclear space, with the distribution varying depending on expression levels.
