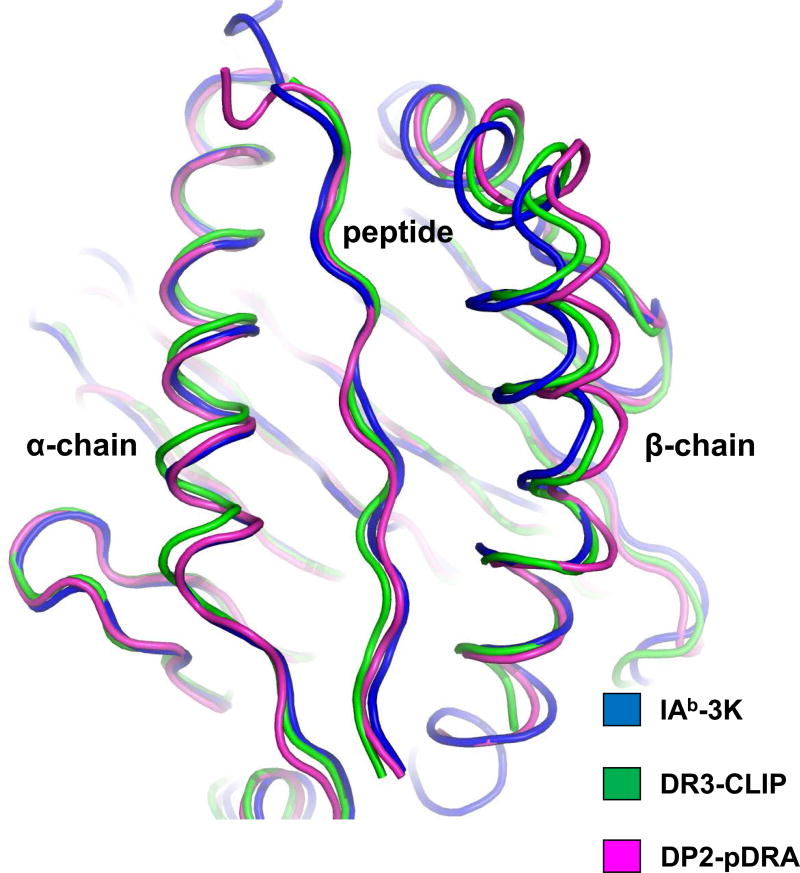
Figure 1. HLA-DP2 possesses a widened peptide binding groove.
Three MHCII structures were overlaid on the basis of their α1 domains: DP2-pDRA (PDB ID code 3LQZ, magenta), HLA-DR3 bound to the invariant chain CLIP peptide (PDB ID code 1A6A, green) and mouse IAb bound to 3K peptide (PDB ID code 1LNU, blue). Human or mouse MHCII with a bound peptide were analyzed for the distance (Å) between the Cα of the p5 amino acid position of the peptide and the Cα of the MHCII β71 amino acid (equivalent to β69 of DP2): 7.62 Å (IAb-p3K), 9.00 Å (DR3-pCLIP), and 10.94 Å (DP2-pDRA). The distance between the peptide and DP2 β-chain α-helix is among the widest of all MHCII molecules.