Abstract
Using a combination of homozygosity mapping and whole-exome sequencing, we identified a novel missense c.1819G>A mutation (S607G) in the endothelin-converting enzyme-like 1 (ECEL1) gene in a consanguineous pedigree of Turkish origin presenting with a syndrome of camptodactyly, scoliosis, limited knee flexion, significant refractive errors and ophthalmoplegia. ECEL1 mutations were recently reported to cause recessive forms of distal arthrogryposis. This report expands on the molecular basis and the phenotypic spectrum of ECEL1-associated congenital contracture syndromes.
Keywords: Arthrogryposis, contractures, camptodactyly, ophthalmoplegia
Introduction
Arthrogryposis multiplex congenita (AMC; OMIM 108110), is a heterogeneous group of syndromes characterized by congenital contractures of more than two joints in multiple parts of the body.(1) AMC affects both sexes equally with an incidence of 1 in 3000 live births, and both genetic and environmental factors are implicated in its etiology.(2-4) When contractures are non-progressive and affect mainly the distal joints (i.e. hands and feet) with limited involvement of proximal joints, the disorder is known as distal arthrogryposis (DA).(5, 6) Recently, recessive mutations in the endothelin converting enzyme-like 1 (ECEL1) gene were reported in patients with distinct forms of distal arthrogryposis (Table 1).(7, 8)
Table 1.
Phenotypes of patients with ECEL1 mutations
| Dieterich et al. | McMillin et al. | Current report | |
|---|---|---|---|
| Inheritance | Recessive | Recessive | Recessive |
| Sex M/F | 5/5 | 5/4 | 1/1 |
| Eye | |||
| Ptosis | 7/10 | 8/9 | 1/2 |
| Ophthalmoplegia | 1/10 | 0/9 | 2/2 |
| Facial involvement | |||
| Arched eye brows | ND | (+) | (+) |
| Bulbous nose | ND | 9/9 | 2/2 |
| Small mouth | 3/10 | ND | 2/2 |
| Micrognathia | ND | 8/9 | 2/2 |
| Decreased facial expression | 3/7 | 1/9 | 2/2 |
| Neck | |||
| Short neck | 10/10 | 4/7 | 2/2 |
| Webbed neck | ND | 3/8 | 2/2 |
| Neck contractures | ND | 4/4 | 2/2 |
| Upper limbs | |||
| Hand and/or finger contractures |
10/10 | 9/9 | 2/2 |
| Wrist contractures | ND | 9/9 | 2/2 |
| Elbow contractures | 3/7 | 5/5 | 0/2 |
| Shoulder contractures | 2/8 | 6/6 | 1/2 |
| Lower limbs | |||
| Foot and/or ankle contractures | 9/10 | 9/9 | 0/2* |
| Knee contractures | |||
| Limited extension | 0/10 | 3/8 | 0/2 |
| Limited flexion | 10/10 | 5/8 | 2/2 |
| Hip contractures | 9/9 | 9/9 | 0/2 |
| Spine | |||
| Scoliosis | 7/10 | 2/9 | 2/2 |
| Hyperlordosis | 9/9 | ND | 1/2 |
| Decreased Muscle mass | 10/10 | ND | 2/2 |
M denotes male, F denotes female, ND denotes no data.
Patients had bilateral pes cavus and overlapping toes.
We studied a Turkish consanguineous pedigree with two affected siblings presenting with a unique AMC syndrome of bilateral camptodactyly, scoliosis, limited knee flexion, pes cavus, ophthalmoplegia, and myopic astigmatism with high cylinder component. The findings in the affected individuals did not meet the major criteria for the diagnosis of DA, and had previously been reported as the second of three families classified with the MIM syndrome “Camptodactyly, myopia, and fibrosis of the medial rectus muscle of eye” (OMIM 602612).(9) We now report that homozygosity-mapping and whole-exome sequencing revealed a novel homozygous missense mutation in ECEL1 that segregated appropriately within the family. The two siblings differ from the recently reported patients harboring ECEL1 mutations because of their significant ophthalmoplegia, refractive errors, and less pronounced contractures of feet or ankles.
Subjects and Methods
Patients
Members of a consanguineous Turkish family (pedigree UP, Figure 1A) with two affected children (IV:2, IV:3) were enrolled in a genetic study of strabismus approved by Boston Children’s Hospital and Pamukkale University Institutional Review Boards. Informed consent was obtained from participating family members. Investigations were conducted in accordance with the principles of the Declaration of Helsinki. IV:2 and IV:3 underwent ophthalmic, neuromuscular, nerve conduction and electromyography (EMG) studies at the age of 30 and 28 years, respectively. A previous report described the phenotypes of IV:2 and IV:3 when they were 13 and 11 years old.(9) At that time, participating family members underwent ophthalmic, neuromuscular and orthopedic examinations, IV:2 and IV:3 underwent body X-rays, brain computerized tomography (CT) and brain and orbital magnetic resonance imaging (MRI), and medial rectus muscle biopsies, and IV:2 underwent electroretinography (ERG).
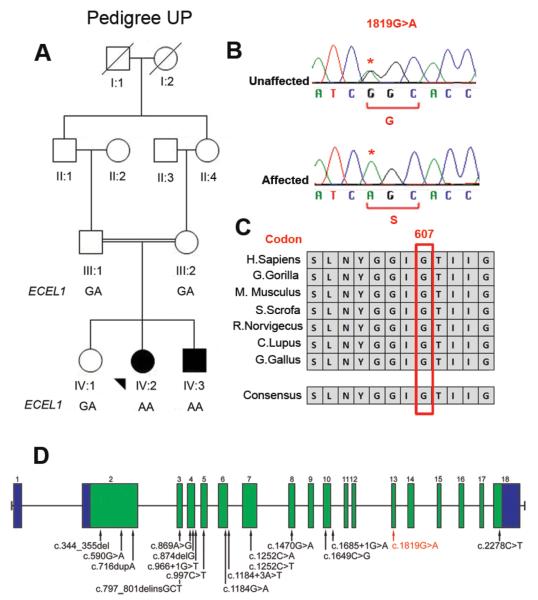
Figure 1. Pedigree structure and mutation analysis.
(A) Schematic of pedigree UP with genotypes of ECEL1 c.1819G>A shown under participants. Squares, males; circles, females; filled symbol, affected. (B) Sanger sequencing chromatograms from an unaffected family member (heterozygous) (top), and from affected individual (homozygous) (bottom) for ECEL1 c.1819G>A substitution. Wild-type and predicted amino acid substitutions are provided below each sequence. (C) Evolutionary conservation of ECEL1 glycine 607 in 7 species. (D) Genomic structure and ECEL1 mutations previously reported to cause DA indicated by black arrows.(7, 8) ECEL1 is composed of 18 exons that encode UTRs (blue) and protein-coding sequences (green). The red arrow indicates the c.1819G>A mutation.
DNA sampling
DNA was extracted from the peripheral blood of participants using the Puregene kit (Qiagen, Valencia, CA).
Homozygosity mapping
Genotyping was performed using Human Mapping Nspl 250K arrays (Affymetrix, Santa Clara, CA), according to the manufacturer’s instructions with an average call rate of >95%. Genome-wide homozygosity analysis was performed, using the software VIGENOS (Visual Genome Studio, Hemosoft Inc, Ankara).(10, 11)
Whole Exome sequencing (WES) and variant validation
Exome sequencing was conducted using 3 μg of genomic DNA from individual UP IV:2 and processed with the SureSelect Human All Exon Kit v.1 (Agilent Technologies, Santa Clara, CA).(12) Sequencing, alignment, variant calling, and annotation followed by filtering and ranking of high quality variants were conducted as previously described.(13)–Putative mutations and appropriate segregation were confirmed by Sanger sequencing.
Results
Clinical evaluation
When reported at 13- and 11-years of age, IV:2 and IV:3 were noted to have characteristic facial features (Table 1). They also had ophthalmoplegia with (IV:2) or without (IV:3) ptosis, compound myopic astigmatism, camptodactyly, scoliosis, limited knee flexion, and pes cavus with overlapping toes. They were not felt to meet diagnostic criteria for DA because they lacked significant foot contractures.(9) Both sibs underwent strabismus surgery at the ages of 10 and 8 respectively with positive forced duction testing noted (Table 1 supplement).
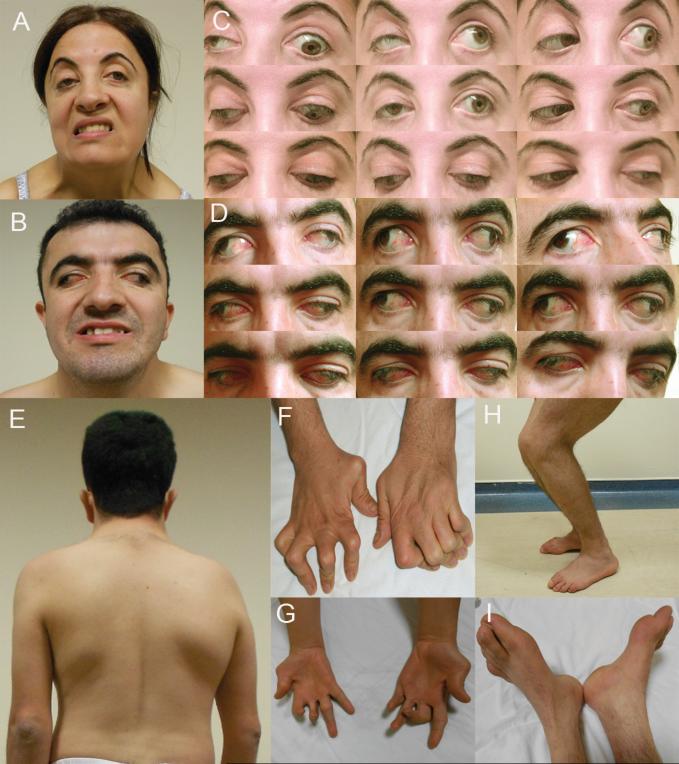
Recent clinical re-evaluation revealed normal cognition and motor development. On examination in primary gaze while fixing with the right eye, IV:2 had 20Δ of exotropia and 5Δ of hypotropia OS. Her right eye was ptotic with limited elevation in abduction and adduction and mild limitation of horizontal movements. Her left eye had no ptosis, but vertical movements were limited on upgaze, with marked adduction and moderate abduction deficits. Downshoot of the left eye occurred on attempted adduction (Figure 2C). IV:3 had divergent strabismus fixus with both eyes frozen in extreme abduction with absent vertical and horizontal movements without ptosis (Figure 2D). Apart from compound myopic astigmatism with high cylinder component (Supplement Table 1), their remaining ophthalmological examinations, including the optic nerve and retinal examination, were normal (Figure 2A&B).
Figure 2. Clinical phenotypes.
A and B: Faces of UP IV:2 (A) and IV:3 (B) attempting to show their teeth; notice facial asymmetry with decreased expression. C and D: Ocular position and motility in 9 directions of gaze in IV:2 (C) and IV:3 (D). IV:2 has ptosis OD and marked limitation of adduction and elevation OS. IV:3 has extreme exotropia and failure of eye movements in all directions. E: Left thoracolumbar scoliosis in IV:3. F: Bilateral metatarsal and interphalangeal extension and flexion contractures. G: Prominent hypothenar and intrinsic hand muscle atrophy. H: Limited knee flexion. I: Bilateral pes cavus and overlapping toes.
There had been no progression in skeletal deformities since the family was initially reported (Figure 2E-I, Table 1, Table 1 supplement). Muscle strength was normal except for bilateral lower motor neuron facial paralysis in both siblings and weakness of muscles of the right hand of IV:2 (2/5) and the left arm of IV:3 (2/5). Nerve conduction and EMG studies including median, ulnar, tibial and peroneal nerves revealed normal motor and sensory conduction velocities. No pathological findings were detected with needle EMG studies of thenar, hypothenar, gastrocnemius and quadriceps muscles.
Genetic analysis
We identified multiple regions of homozygosity shared only by the two affected siblings. The largest were 14Mb and 20Mb on chromosomes 2 and 12, respectively, and contained hundreds of genes. Thus, we performed WES for individual IV:2 to identify causative variants. We obtained mean coverage of 86% at 10X resulting in ~18000 variants. We investigated the novel homozygous variants within the shared regions of homozygosity. This analysis resulted in 5 variants, one of which was the missense change c.1819G>A in ECEL1 gene, predicted to substitute a serine for glycine at the highly conserved residue 607 (p.G607S) which falls in the predicted α24 helix in ECEL1 lumenal domain (Figure 1B,C&D). No other variant fell within a known distal arthrogryposis gene. The variant was absent from 468 ethnically-matched exomes, segregated appropriately within the family (Figure 1A&B), and was predicted to be damaging by in silico analysis (14-16).
Discussion
ECEL1 mutations were recently reported by McMillin et al. and by Dieterich et al. in individuals with distal arthrogryposis.(7, 8) McMillin et al. identified ECEL1 mutations in individuals with a phenotype of ptosis, distinct facial features, severe hand camptodactyly, toe and foot contractures, and knee deformities.(7) McMillin classified these families as DA5D, based on the presence of ptosis but not ophthalmoplegia.(7) Similarly, Dieterich et al. identified ECEL1 mutations in individuals with a syndrome of DA characterized by ptosis, tongue atrophy, camptodactyly, limited knee flexion, and feet deformities. Ocular motility was abnormal in only one patient with Duane syndrome.(8) Five patients developed restrictive pulmonary disease.(8)
The two siblings in this report share similar facial features with the reported patients, (Table 1 and Supplementary Table 1), while they differ because they have significant ophthalmoplegia, refractive errors, and severe camptodactyly involving the fingers, while they lack major feet or ankle contractures and respiratory compromise.(7, 8)
ECEL1 is a member of the neprilysin family of zinc metalloendopeptidases and is highly expressed in neurons within the central and peripheral nervous system in humans and rodents.(8, 17-19) Ecel1−/− mice die soon after birth due to respiratory failure. They have a pathological decrease in final branching of nerve terminals to the diaphragm and skeletal muscles, and failure of formation of an adequate number of neuromuscular junctions (NMJs).(18, 20) While the molecular mechanism underlying this phenotype remains undefined, zinc metalloendopeptidases such as ADAMs and matrix metalloproteases may directly control axon growth by cleaving axon guidance receptors and ligands.(21) Alternatively, ECEL1 could bind to factors required for the final axonal branching as seen with Meltrin β, a metallopeptidase that binds to EphA4 and regulates ephrinA4 signaling required for NMJ formation.(22)
The significant congenital ophthalmoplegia reported here, together with reports of expression of ECEL1 in embryonic spinal and cranial motor neurons of rats,(19) raises the possibility that ECEL1 mutations can result in a new genetic form of the congenital cranial dysinnervation disorders (CCDDs). CCDDs are congenital neuromuscular diseases resulting from developmental errors in innervation of cranial musculature in humans,(23) and a subset are caused by mutations in genes important for axon growth and guidance.(24) Notably, the phenotype of the siblings in this report overlaps with the phenotype resulting from a subset of missense mutations in the neuronal-specific TUBB3 gene.(25)
Significant refractive errors have not been reported with ECEL1 mutations.(7, 8) ECEL1 is, however, expressed in the developing retina, (19) and may play a role in control of ocular growth and refraction.(26, 27) Moreover, another form of AMC, Escobar syndrome (OMIM 609339), results from homozygous mutations in the cholinergic receptor nicotinic gamma gene, which was recently reported to also be associated with refractive errors.(28, 29) These findings are intriguing given that retinal cholinergic signaling has been postulated as a mechanism through which retina affects refractive development.(27, 30) Investigating refraction in other ECEL1-mutation-positive patients might support an unrevealed role of ECEL1 in ocular development and cholinergic signaling.
Supplementary Material
Acknowledgments
We thank members of pedigree UP for their participation. This study was supported by the Al-Habtoor Dubai-Harvard Foundation fellowship to SS, the Manton Center for Orphan Disease Research to ECE, and National Institute of Health R01EY12498, MRRC grant P30 HD018655 and was partially supported by Pamukkale University Scientific Research Unit (2006 TPF 002). E.C.E. is a Howard Hughes Medical Institute Investigator.
Footnotes
Authors’ conflict of interest statement:
Sherin Shaaban:
The author declares no conflict of interest.
Fusun Duzcan:
The author declares no conflict of interest.
Cem Yildirim:
The author declares no conflict of interest.
Wai-man Chan:
The author declares no conflict of interest.
Caroline Andrews:
The author declares no conflict of interest.
Nurten Akarsu
The author declares no conflict of interest.
Elizabeth Engle: The author declares no conflict of interest.
Suggested referees:
1- Monique Ryan: University of Melbourne, Australia
monique.ryan@rch.org.au
2- Birgit Lorenz: Universitätsklinikum Giessen and Marburg GmbH, Germany
birgit.lorenz@uniklinikum-giessen.de
3- Christopher Inglehearn: Leeds University, UK
c.inglehearn@leeds.ac.uk
4- Ferda Percin: Gazi University, Turkey
ferdaep@yahoo.com
References
- 1.Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6:159–166. [PubMed] [Google Scholar]
- 2.Navti OB, Kinning E, Vasudevan P, et al. Review of perinatal management of arthrogryposis at a large UK teaching hospital serving a multiethnic population. Prenat Diagn. 2010;30:49–56. doi: 10.1002/pd.2411. [DOI] [PubMed] [Google Scholar]
- 3.Kalampokas E, Kalampokas T, Sofoudis C, et al. Diagnosing arthrogryposis multiplex congenita: a review. ISRN Obstet Gynecol. 2012;2012:264918. doi: 10.5402/2012/264918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamshad M, Van Heest AE, Pleasure D. Arthrogryposis: a review and update. J Bone Joint Surg Am. 2009;91(Suppl 4):40–46. doi: 10.2106/JBJS.I.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamshad M, Jorde LB, Carey JC. A revised and extended classification of the distal arthrogryposes. Am J Med Genet. 1996;65:277–281. doi: 10.1002/(SICI)1096-8628(19961111)65:4<277::AID-AJMG6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Hall JG, Reed SD, Greene G. The distal arthrogryposes: delineation of new entities--review and nosologic discussion. Am J Med Genet. 1982;11:185–239. doi: 10.1002/ajmg.1320110208. [DOI] [PubMed] [Google Scholar]
- 7.McMillin MJ, Below JE, Shively KM, et al. Mutations in ECEL1 Cause Distal Arthrogryposis Type 5D. Am J Hum Genet. 2012 doi: 10.1016/j.ajhg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieterich K, Quijano-Roy S, Monnier N, et al. The neuronal endopeptidase ECEL1 is associated with a distinct form of recessive distal arthrogryposis. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds514. [DOI] [PubMed] [Google Scholar]
- 9.Kilic I, Kilic BA, Ergin H, et al. Camptodactyly, myopia, and fibrosis of the medial rectus of the eye in two sibs born to consanguineous parents: autosomal recessive entity? Am J Med Genet. 1998;77:28–30. doi: 10.1002/(sici)1096-8628(19980428)77:1<28::aid-ajmg7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Kayserili H, Uz E, Niessen C, et al. ALX4 dysfunction disrupts craniofacial and epidermal development. Hum Mol Genet. 2009;18:4357–4366. doi: 10.1093/hmg/ddp391. [DOI] [PubMed] [Google Scholar]
- 11.Uz E, Alanay Y, Aktas D, et al. Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal-recessive ALX-related frontonasal dysplasia. Am J Hum Genet. 2010;86:789–796. doi: 10.1016/j.ajhg.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnirke A, Melnikov A, Maguire J, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol. 2009;27:182–189. doi: 10.1038/nbt.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb BD, Shaaban S, Gaspar H, et al. HOXB1 Founder Mutation in Humans Recapitulates the Phenotype of Hoxb1(−/−) Mice. American Journal of Human Genetics. 2012;91:171–179. doi: 10.1016/j.ajhg.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods: 7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-Costa C, Gelpi JL, Zamakola L, et al. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics. 2005;21:3176–3178. doi: 10.1093/bioinformatics/bti486. [DOI] [PubMed] [Google Scholar]
- 17.Valdenaire O, Richards JG, Faull RL, et al. XCE, a new member of the endothelin-converting enzyme and neutral endopeptidase family, is preferentially expressed in the CNS. Brain Res Mol Brain Res. 1999;64:211–221. doi: 10.1016/s0169-328x(98)00321-0. [DOI] [PubMed] [Google Scholar]
- 18.Nagata K, Kiryu-Seo S, Maeda M, et al. Damage-induced neuronal endopeptidase is critical for presynaptic formation of neuromuscular junctions. J Neurosci. 2010;30:6954–6962. doi: 10.1523/JNEUROSCI.4521-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata K, Kiryu-Seo S, Kiyama H. Localization and ontogeny of damage-induced neuronal endopeptidase mRNA-expressing neurons in the rat nervous system. Neuroscience. 2006;141:299–310. doi: 10.1016/j.neuroscience.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer A, Valdenaire O, Koster A, et al. Neonatal lethality in mice deficient in XCE, a novel member of the endothelin-converting enzyme and neutral endopeptidase family. J Biol Chem. 1999;274:20450–20456. doi: 10.1074/jbc.274.29.20450. [DOI] [PubMed] [Google Scholar]
- 21.Bai G, Pfaff SL. Protease regulation: the Yin and Yang of neural development and disease. Neuron. 2011;72:9–21. doi: 10.1016/j.neuron.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yumoto N, Wakatsuki S, Kurisaki T, et al. Meltrin beta/ADAM19 interacting with EphA4 in developing neural cells participates in formation of the neuromuscular junction. PLoS One. 2008;3:e3322. doi: 10.1371/journal.pone.0003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutowski NJ, Bosley TM, Engle EC. 110th ENMC International Workshop: The congenital cranial dysinnervation disorders (CCDDs) Naarden, The Netherlands, 25-27 October, 2002. Neuromuscul Disord. 2003;13:573–578. doi: 10.1016/s0960-8966(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 24.Oystreck DT, Engle EC, Bosley TM. Recent progress in understanding congenital cranial dysinnervation disorders. J Neuroophthalmol. 2011;31:69–77. doi: 10.1097/WNO.0b013e31820d0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tischfield MA, Baris HN, Wu C, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Stone RA, McGlinn AM, Baldwin DA, et al. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci. 2011;52:5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann K, Muller JS, Stricker S, et al. Escobar syndrome is a prenatal myasthenia caused by disruption of the acetylcholine receptor fetal gamma subunit. Am J Hum Genet. 2006;79:303–312. doi: 10.1086/506257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashby R, McCarthy CS, Maleszka R, et al. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp Eye Res. 2007;85:15–22. doi: 10.1016/j.exer.2007.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.