Fig. 1.
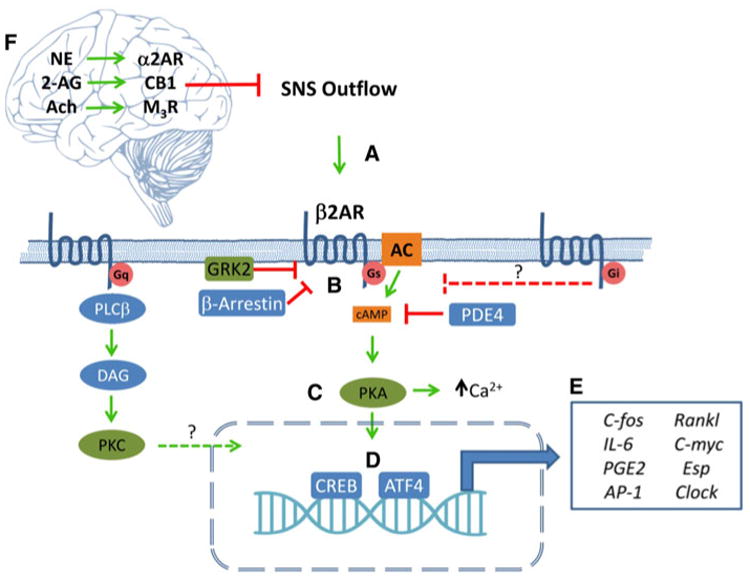
The β2AR is the central mediator of SNS signaling in osteoblasts. (a) Activation of the SNS releases catecholamines in the bone which activate osteoblastic β2AR. (b) Signaling is primarily mediated by the stimulatory G-alpha subunit (Gs), which activates adenylyl cyclase, resulting in increased intracellular cAMP, which then activates protein kinase A. (c) The active PKA catalytic subunits can then phosphorylate cytosolic proteins to increase intracellular calcium or translocate to the nucleus (d), where they activate CREB and ATF4. (e) These transcription factors modulate the expression of various genes within the osteoblast that affect its own function as well as the behavior of other cells, e.g., osteoclasts, within the bone. Multiple proteins such as arrestins, phosphodiesterases, and GPCR kinases have been shown to modulate β2AR transduction at different steps within the osteoblast. Additionally, other neurotransmitter receptors that signal through Gq likely also interact with β2AR, signaling transduction within the nucleus. Gi-coupled receptors may be involved in controlling the magnitude of β2AR effects along with other Gs-coupled receptors. (f) β2AR signaling in bone is also controlled centrally by signaling through brain-expressed adrenergic, cannabinoid, and muscarinic receptors, whose activation decreases SNS outflow
