Abstract
Purpose
The objective of this study was to evaluate the histologic remodeling profile and biomechanical properties of the porcine abdominal wall after repair with HDMI-crosslinked (Permacol®) or non-crosslinked (Strattice®) porcine dermis in a porcine model of ventral hernia repair.
Methods
Bilateral incisional hernias were created in Yucatan minipigs and repaired after 21 days. The repair site, including mesh and abdominal wall, was harvested after 1, 6, and 12 months and subjected to histologic analysis and uniaxial testing. Native abdominal wall without mesh was also subjected to uniaxial tensile testing.
Results
Permacol® demonstrated significant improvement over time in every remodeling category except scaffold degradation, while remodeling characteristics of Strattice® remained relatively unchanged over time for every category except fibrous encapsulation and neovascularization. However, remodeling scores for Strattice® were already significantly higher after just 1 month compared to Permacol® in the categories of cellular infiltration, ECM deposition, and neovascularization, providing evidence of earlier remodeling of the non-crosslinked grafts compared to the crosslinked grafts. The tensile strength and stiffness of both crosslinked and non-crosslinked graft-tissue composites were greater than the tensile strength and stiffness of the native porcine abdominal wall in the very early post-operative period (1 month), but there was no difference in tensile strength or stiffness by the end of the study period (12 months).
Conclusions
HDMI collagen crosslinking of porcine dermis scaffolds reduces the early histologic remodeling profile but does not significantly impact the tensile strength or stiffness of the graft-tissue composites in a porcine model of ventral hernia repair.
Keywords: Biologic mesh, Crosslinking, Porcine, Remodeling, Tensile strength, Ventral hernia repair
Introduction
Biologic mesh reinforcement of abdominal wall hernia repair sites provides a viable alternative to autologous tissue transfer and primary suture repair in clean contaminated or contaminated surgical sites. Preclinical evidence indicating that biologic meshes enable revascularization of soft tissue repair sites and improve pathogen clearance in contaminated and infected surgical sites [1, 2], and clinical evidence demonstrating that biologic meshes do not necessarily require removal when infected [3–5] are contributing to the rising demand for biologic scaffolds in this clinical setting. Despite the growing market of commercially available biologic scaffolds for soft tissue repair, scant data are available regarding the distinguishing characteristics of these biologic scaffolds, and the potential impact these scaffold properties may have on clinical outcomes.
Biologic scaffolds possess varying degree of biocompatibility and biodegradability based on their molecular compositions. The molecular composition of a biologic scaffold results from the genetic and phenotypic characteristics of the graft’s native species, donor, and tissue type, as well as the biochemical and mechanical processing to which the graft is subjected during commercial manufacture [6–9]. Ideally, manufacturing processes should achieve decellularization, enhancement, sterilization, and preservation of the grafts for storage while minimizing disruption of the native scaffold structures. Manufacturing processes that alter the molecules of the native scaffold may lead to more rapid in vivo degradation of the scaffold, a heightened host inflammatory cell response, and/or fibrotic encapsulation by host fibroblasts. By preventing host cell infiltration and host neovascularization, these processes ultimately favor scar formation rather than constructive tissue remodeling in the host [6]. Manufacturing processes can therefore have a substantial impact on the host tissue response, the constructive remodeling of the soft tissue, and the integrity of the tissue repair. Many of these manufacturing processes remain proprietary, thereby restricting independent scientific investigation of their potential impact on clinical outcomes after soft tissue repair with biologic scaffolds.
Collagen crosslinking is one of these manufacturing processes. Crosslinking is a biochemical process that results in the creation of bonds between the collagen triple helices of the biologic scaffold and is believed to enhance the strength and durability of the scaffold. Some manufacturers of biologic scaffolds intentionally crosslink the collagen helices to stabilize the scaffold to resist enzymatic collagen degradation by matrix metalloproteinases in vivo. However, collagen crosslinking may also occur as an unintended consequence of other manufacturing processes, including scaffold radiation for sterilization and scaffold dehydration for long-term preservation. Regardless of whether the collagen crosslinking is an intentional or an unintentional consequence, these biochemical processes may create short and inflexible bonds that restrict early cellular infiltration and can leave residual chemical byproducts that elicit an inflammatory response [6–11].
Despite widespread clinical use, the role of collagen crosslinking in the in vivo remodeling of the biologic scaffold and the biomechanical integrity of the soft tissue repair site remains to be proven. The objective of the current study is to compare the histologic remodeling profile and biomechanical properties of the porcine abdominal wall after repair with crosslinked (Permacol®) or non-crosslinked (Strattice®) porcine dermis over a 1-year period in a porcine model of ventral hernia repair. We hypothesize that hernia sites repaired with crosslinked biologic mesh will demonstrate less remodeling and greater tensile strength and stiffness than hernia sites repaired with non-crosslinked biologic mesh in a porcine model of ventral hernia repair.
Materials and methods
Animal care and material selection
This study was performed under strict compliance with a protocol approved by the Animal Studies Committee of Washington University School of Medicine. 36 female Yucatan minipigs were acclimated for 72 h in our animal facility, housed, fed, and handled as prescribed by the Guide for the Care and Use of Laboratory Animals [12] and standard protocols for humane animal treatment maintained by the Washington University Division of Comparative Medicine. Strict adherence to sterility was enforced for all procedures. The biologic scaffolds selected for this comparative study were chosen for their shared species and tissue type origin, and the distinguishing feature of either the presence of absence of proprietary treatments for intentional collagen crosslinking during manufacture: Permacol® Surgical Implant (Covidien Surgical; Norwalk, CT; crosslinked porcine dermis), and Strattice® Reconstructive Tissue Matrix (LifeCell Corporation; Branchburg, NJ; non-crosslinked porcine dermis). Permacol® Surgical Implant is an intentionally crosslinked porcine dermis scaffold. Following acetone and buffered trypsin baths to eliminate cells and immunogenic agents from the scaffold, a non-calcifying hexamethylene diisocyanate (HDMI) treatment is used to crosslink the collagen extracellular matrix. The scaffold is then sterilized with gamma irradiation [13–15]. Strattice® Reconstructive Tissue Matrix (LifeCell Corporation; Branchburg, NJ) is a porcine dermis scaffold that is not intentionally cross-linked. Following a proprietary process to remove cells and immunogenic agents from the extracellular matrix, the scaffold is sterilized with electron beam irradiation and dehydrated for preservation during storage [14–16]. Permacol® and Strattice® are both approved by the United States Food and Drug Administration (FDA) for soft tissue repair applications, including but not limited to abdominal wall reconstruction. Manufacturer-supplied instructions for use were followed for the storage and handling of all biologic scaffolds.
Ventral hernia repair model
A model of ventral hernia repair was utilized as previously described [17–19]. In brief, bilateral abdominal wall defects were created in Yucatan minipigs which were allowed to mature for 21 days before repair. Abdominal wall defects then underwent preperitoneal repair with either Permacol® or another mesh type in the previously published study [19], or with Strattice® for both defects in the current study. Eight minipigs repaired with Permacol® and four minipigs repaired with Strattice® were subsequently euthanized at each time point (1, 6, and 12 months). Therefore, a total of 8 Permacol® graft-tissue composites and 8 Strattice® graft-tissue composites were procured at each time point. Histologic and biomechanical property analysis of the graft-tissue composites ensued.
Creation of ventral hernia defects
Peri-operative antibiotic prophylaxis was provided with intramuscular cefazolin (15–25 mg/kg), and peri-operative analgesia was provided with intramuscular buprenorphine (0.02–0.05 mg/kg) and intramuscular caprofen (2.2–4.4 mg/ kg) for each pig. Anesthestic induction using intramuscular ketamine (2.2 mg/kg), xylazine (2.2 mg/kg), and telazol (4.4 mg/kg) was followed by endotracheal intubation and general anesthesia maintenance with 3 % isoflurane. Hydration was provided by intravenous saline throughout the procedure. The pig was positioned supine, and the abdominal skin prepped with 10 % povidone-iodine solution. Strict sterile conditions were maintained for each procedure. Bilateral abdominal wall defects measuring 4 cm on the cranial-caudal axis were made using sharp dissection through the skin, subcutaneous fat, aponeurotic muscle, and fascial layers of the abdominal wall until the peritoneum was reached. The peritoneum was not violated. The abdominal wall muscular and fascial defects were left open, while the subcutaneous tissue and skin of the incisions were reap-proximated with a double layer of 3–0 polydioxanone (PDS) suture and sealed with cyanoacrylate-based dermal glue. Dermal glue provided 72-h post-operative barrier protection from fluid and fecal contamination. Antibiotic prophylaxis of oral cephalexin (25–35 mg/kg) was administered for 5 consecutive post-operative days. Analgesia included buprenorphine (0.02–0.05 mg/kg IM) in the immediate postoperative period and caprofen (4.4 mg/kg IM q12 h or 2.2 mg/kg po q12 h) as needed thereafter.
Repair of ventral hernia defects
After 21 post-operative days, the matured hernia defects underwent retromuscular repair. Each of the incisional ventral hernia defects was repaired with an 8 cm × 10 cm graft of either Permacol® or Strattice®. Following preoperative treatment and general anesthesia induction as described for the procedure during which the hernia defects were created, the skin and subcutaneous tissue of the previously described bilateral incisions were opened using sharp dissection to expose the hernia defects. The biologic scaffolds were oriented with the long edge (10 cm) running axially and the short edge (8 cm) running transversely beneath the abdominal wall defects in the preperitoneal retromuscular space. Each biologic scaffold was secured in position with 8 circumferential transfascial interrupted #0 prolene sutures placed approximately 3 cm apart and at least 1 cm from the scaffold edge. An overlap of 2–3 cm was achieved circumferentially between the scaffold-abdominal wall interface (Fig. 1). To eliminate excess dead space, the hernia defect was closed with interrupted #0 PDS. The subcutaneous tissue and skin were reapproximated using a double layer of interrupted 3–0 PDS suture and sealed with cyanoacrylate-based dermal glue. The post-operative care was similar to that following the procedure to create the hernia defects.
Fig. 1.
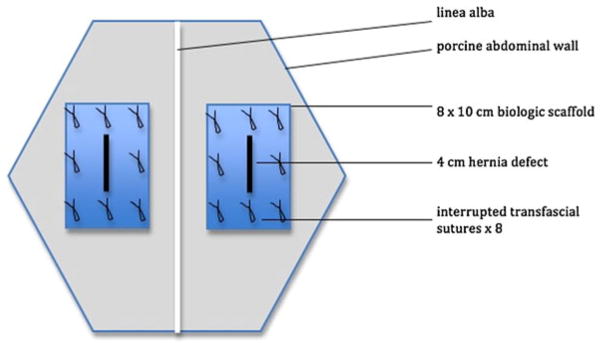
Schematic of bilateral open ventral hernia on porcine abdominal wall
Euthanasia and procurement of specimens
Following a survival of 1, 6, or 12 months, the minipigs were euthanized for retrieval of the biologic scaffold-abdominal wall tissue composite. Eight minipigs repaired with Permacol® and four minipigs repaired with Strattice® were euthanized at each time point. Each minipig was sedated with intramuscular telazol (4.4 mg/kg), xylazine (2.2 mg/kg), and ketamine (2.2 mg/kg). After achievement of adequate anesthesia, euthanasia was performed by administration of intravenous phenobarbital (>100 mg/kg) or intravenous potassium chloride (>100 mg/kg). A 15 cm midline laparotomy incision was made, and the biologic scaffolds were inspected for in situ appearance. The biologic scaffold-abdominal wall tissue composite was harvested en bloc from each animal and trimmed in thickness to include only a single muscle layer, the peritonealized transversus abdominis muscle layer. A 4 cm × 4 cm composite specimen from the center of each repair site was recovered and portioned into a 1 cm × 4 cm composite strip for histologic analysis and a 3 cm × 4 cm composite strip for biomechanical analysis.
Histology
For histologic analysis, the 1 cm × 4 cm scaffold-tissue composite was embedded in paraffin. Thin sections were stained with hematoxylin and eosin and visualized by light microscopy at 40×, 100×, and 200× magnification by a veterinary pathologist. Five to ten non-overlapping fields per specimen were analyzed at 100× magnification and evaluated for cellular infiltration, cell types present, extracellular matrix deposition, scaffold degradation, fibrous encapsulation, and neovascularization, according to a scale adapted from Valentin et al. [20] and previously published for similar applications (Table 1) [17–19]. After reviewing these 5–10 fields per specimen, a single score was assigned for each remodeling category. More favorable outcomes of scaffold remodeling are represented by higher grades on the scale: greater degree of cellular infiltration, greater scaffold degradation, greater deposition of extracellular matrix, greater degree of neovascularization, less inflammatory cell response, and less encapsulation. The mean of these six remodeling category scores was calculated to give the composite histologic score for each specimen.
Table 1.
Histologic Scoring System
| Histologic scoring system | Score
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Cellular infiltration | Zero cells in contact with mesh | Cells contact periphery, no infiltration into mesh | Cells infiltrate mesh, but none reach center | Cells penetrate into center of mesh |
| Cell types (Inflammatory cells include neutrophils, macrophages, and foreign body giant cells) | Inflammatory cells present no fibroblasts | Primarily inflammatory cells, few fibroblasts | Primarily fibroblasts, few inflammatory cells | Fibroblasts only, no inflammatory cells |
| Inflammatory response | Mononuclear and foreign body giant cells | Primarily mononuclear cells | Few mononuclear cells | Zero inflammatory cells present |
| Host extracellular matrix (ECM) deposition | No host ECM deposition | Host ECM deposited at periphery of mesh | Host ECM deposited around individual mesh fibers but not bridging across mesh interstices | Host ECM deposited within mesh interstices and bridging between mesh fibers |
| Neovascularization | Zero blood vessels present | Vessels present at mesh periphery only, no vessels present within mesh interstices | Vessels present within mesh interstices but not bridging across mesh interstices | Vessels present within mesh interstices and bridging mesh interstices, forming an interconnected microvascular network |
| Fibrosis | Scar plate fully embedding entire mesh including fibers and interstices | Fibrosis surrounding individual mesh fibers and bridging across mesh interstices in some places | Fibrosis surrounding individual mesh fibers but not bridging across mesh interstices | No fibrosis |
| Fibrous encapsulation | Measurement of thickness rather than a score | |||
Tensiometry
For tensiometry, the 3 cm × 4 cm scaffold-tissue composite was oriented vertically and secured in the grips of an Instron Series 5542 Universal Testing System (Instron, Norwood, MA). Each specimen was tested to failure at a rate of 0.42 mm/s (1 inch/min), and the maximum load sustained by the specimen was recorded in Newtons (N). The tensile strength per unit width was calculated by dividing the maximum sustained load by the width of the specimen (N/cm). The stiffness (N/mm) of the specimen was approximated by calculating the slope of the force versus displacement curve in the linear portion of the curve between 30 and 70 % of the maximal force. For comparison of the biomechanical properties, specimens of the native abdominal wall away from the soft tissue repair site were evaluated in the same fashion.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). The 8 Permacol® graft-tissue composite specimens at each time point were each procured from different pigs and therefore treated as independent specimens; whereas, 2 Strattice® graft-tissue composite specimens were procured from each of 4 pigs at each time point, and thus, the mean measurement per pig was used in the analysis. To detect the differences, data were analyzed by the non-parametric Kruskal–Wallis test. All statistical tests were two-sided using an α = 0.05 level of significance. Bonferroni correction was considered as appropriate. SAS version 9.3 (Cary, NC) was used to perform all statistical analyses.
Results
Histology
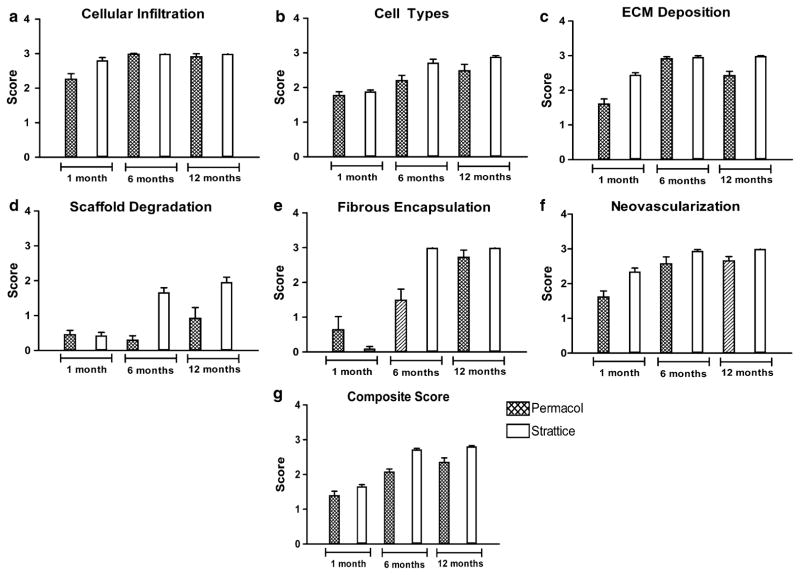
Five to ten non-overlapping fields per specimen were analyzed at 100× magnification and graded on a scale of 0–3 for cellular infiltration, cell types present, extracellular matrix deposition, scaffold degradation, fibrous encapsulation, and neovascularization. From these 5–10 scores per specimen, a single score was then assigned for each remodeling category. Higher grades on the scale represent more favorable outcomes with regard to scaffold remodeling. The mean of these six remodeling category scores was calculated to give the composite histologic score for each specimen.
Cellular infiltration scores
The cellular infiltration scores (Fig. 2a) for explanted Permacol® graft-tissue composites were 2.27 ± 0.15 at 1 month post-implantation, 2.99 ± 0.02 at 6 months post-implantation, and 2.92 ± 0.08 at 12 months post-implantation. The cellular infiltration scores for explanted Strattice® graft-tissue composites were 2.81 ± 0.08 at 1 month post-implantation, 3.00 ± 0.00 at 6 months post-implantation, and 3.00 ± 0.00 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in cellular infiltration scores at 6 and 12 months post-implantation compared to cellular infiltration scores at 1 month post-implantation (p = 0.0020 and p = 0.0035, respectively); however, no significant difference was detected between cellular infiltration scores at 12 months post-implantation compared to cellular infiltration scores at 6 months post-implantation (p = 0.9273). For Strattice® graft-tissue composites, cellular infiltration scores did not change significantly at 6 and 12 months post-implantation compared to cellular infiltration scores at 1 month post-implantation using a Bonferroni correction (p = 0.0472 and p = 0.0472, respectively) or between cellular infiltration scores at 12 months post-implantation compared to cellular infiltration scores at 6 month post-implantation (p = 1.000). However, Strattice® graft-tissue composites did exhibit significantly greater cellular infiltration scores compared to Permacol® graft-tissue composites at 1 month post-implantation (p = 0.0334). No differences were detected between Permacol® graft-tissue composites and Strattice® graft-tissue composites at 6 months (p = 0.4795) or 12 months (p = 0.4795) post-implantation.
Fig. 2.
Histologic scores for H&E stained slides
Cell type scores
The cell type scores (Fig. 2b) for explanted Permacol® graft-tissue composites were 1.78 ± 0.10 at 1 month post-implantation, 2.21 ± 0.14 at 6 months post-implantation, and 2.49 ± 0.18 at 12 months post-implantation. The cell type scores for explanted Strattice® graft-tissue composites were 1.89 ± 0.04 at 1 month post-implantation, 2.72 ± 0.10 at 6 months post-implantation, and 2.89 ± 0.03 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in cell type scores at 12 months post-implantation compared to cell type scores at 1 month post-implantation (p = 0.0157); however, no significant difference was detected between cell type scores at 6 months post-implantation compared to cell type scores at 1 month post-implantation (p = 0.0311), or between cell type scores at 12 months post-implantation compared to cell type scores at 6 months post-implantation (p = 0.1272). Using a Bonferroni correction, Strattice® graft-tissue composites approached but did not reach significant improvement in cell type scores at 6 or 12 months post-implantation compared to cell type scores at 1 month post-implantation (p = 0.0209, p = 0.0209, respectively) and did not demonstrate significance between cell type scores at 12 months post-implantation compared to cell type scores at 6 months post-implantation (p = 0.2482). While there was no significant difference in the cell type scores for Permacol® and Strattice® graft-tissue composites at 1 or 12 months post-implantation (p = 0.3958 and p = 0.0894, respectively), Strattice® graft-tissue composites exhibited significantly greater cell type scores compared to Permacol® graft-tissue composites at 6 months post-implantation (p = 0.0412).
Extracellular matrix (ECM) deposition scores
The ECM deposition scores (Fig. 2c) for explanted Permacol® graft-tissue composites were 1.61 ± 0.14 at 1 month post-implantation, 2.92 ± 0.05 at 6 months post-implantation, and 2.43 ± 0.12 at 12 months post-implantation. The ECM deposition scores for explanted Strattice® graft-tissue composites were 2.45 ± 0.06 at 1 month post-implantation, 2.96 ± 0.04 at 6 months post-implantation, and 2.99 ± 0.01 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in ECM deposition scores at 6 and 12 months post-implantation compared to ECM deposition scores at 1 month post-implantation (p = 0.0006 and p = 0.0046, respectively), and a significant difference was detected between ECM deposition scores at 12 months post-implantation compared to ECM deposition scores at 6 months post-implantation (p = 0.0040). Using a Bonferroni correction, Strattice® graft-tissue composites approached but did not reach significant difference in ECM deposition scores at 6 and 12 months post-implantation compared to ECM deposition scores at 1 month post-implantation (p = 0.0180 and p = 0.0180, respectively) and further did not demonstrate a significant difference between ECM deposition scores at 12 months post-implantation compared to ECM deposition scores at 6 months post-implantation (p = 0.8501). Strattice® graft-tissue composites exhibited significantly greater ECM deposition scores compared to Permacol® graft-tissue composites at 1 and 12 months post-implantation (p = 0.0066 and p = 0.0062, respectively); however, there was no significant difference in the ECM deposition scores for Permacol® graft-tissue composites and Strattice® graft-tissue composites at 6 months post-implantation (p = 0.6134).
Scaffold degradation scores
The scaffold degradation scores (Fig. 2d) for explanted Permacol® graft-tissue composites were 0.46 ± 0.12 at 1 month post-implantation, 0.30 ± 0.12 at 6 months post-implantation, and 0.93 ± 0.30 at 12 months post-implantation. The scaffold degradation scores for explanted Strattice® graft-tissue composites were 0.43 ± 0.09 at 1 month post-implantation, 1.67 ± 0.13 at 6 months post-implantation, and 1.96 ± 0.14 at 12 months post-implantation. Using a Bonferroni correction, no signifi-cant differences in scaffold degradation scores were detected between Permacol® graft-tissue composites at 1 and 6 months post-implantation (p = 0.1709), at 1 and 12 months post-implantation (p = 0.1556), or at 6 and 12 months post-implantation (p = 0.0576). Using a Bonferroni correction, Strattice® graft-tissue composites approached but did not reach significant difference in scaffold degradation scores at 6 and 12 months post-implantation compared to scaffold degradation scores at 1 month post-implantation (p = 0.0209 and p = 0.0209, respectively), and no significant difference was detected between scaffold degradation scores at 12 months post-implantation compared to scaffold degradation scores at 6 months post-implantation (p = 0.0833). While no significant differences were detected in the scaffold degradation scores for Permacol® and Strattice® graft-tissue composites at 1 month post-implantation (p = 0.8649), Strattice® graft-tissue composites exhibited significantly greater scaffold degradation scores compared to Permacol® graft-tissue composites at 6 and 12 months post-implantation (p = 0.0065 and p = 0.0415, respectively).
Fibrous encapsulation scores
The fibrous encapsulation scores (Fig. 2e) for explanted Permacol® graft-tissue composites were 0.65 ± 0.37 at 1 month post-implantation, 1.50 ± 0.31 at 6 months post-implantation, and 2.73 ± 0.20 at 12 months post-implantation. The fibrous encapsulation scores for explanted Strattice® graft-tissue composites were 0.10 ± 0.06 at 1 month post-implantation, 3.00 ± 0.00 at 6 months post-implantation, and 3.00 ± 0.00 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in fibrous encapsulation scores at 12 months post-implantation compared to fibrous encapsulation scores at 1 month post-implantation (p = 0.0016) and between fibrous encapsulation scores at 12 months post-implantation compared to 6 months post-implantation (p = 0.0072). However, no significant difference was detected between fibrous encapsulation scores at 1 and 6 months post-implantation (p = 0.0881). Using a Bonferroni correction, Strattice® graft-tissue composites demonstrated significant improvement in fibrous encapsulation scores at 6 and 12 months post-implantation compared to fibrous encapsulation scores at 1 month post-implantation (p = 0.0132 and p = 0.0132, respectively); however, no significant difference was detected between fibrous encapsulation scores at 12 months post-implantation compared to fibrous encapsulation scores at 6 months post-implantation (p = 1.000). While no significant differences were detected in the fibrous encapsulation scores of Permacol® and Strattice® graft-tissue composites at 1 month (p = 0.5865) and 12 months (p = 0.2963) post-implantation, Strattice® graft-tissue composites exhibited significantly greater fibrous encapsulation scores compared to Permacol® graft-tissue composites at 6 months post-implantation (p = 0.0137).
Neovascularization scores
The neovascularization scores (Fig. 2f) for explanted Permacol® graft-tissue composites were 1.62 ± 0.17 at 1 month post-implantation, 2.58 ± 0.19 at 6 months post-implantation, and 2.66 ± 0.12 at 12 months post-implantation. The neovascularization scores for explanted Strattice® graft-tissue composites were 2.35 ± 0.10 at 1 month post-implantation, 2.94 ± 0.04 at 6 months post-implantation, and 3.00 ± 0.00 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in neovascularization scores at 6 and 12 months post-implantation compared to neovascularization scores at 1 month post-implantation (p = 0.0100 and p = 0.0016, respectively); however, no significant difference was detected between neovascularization scores at 12 months post-implantation compared to neovascularization scores at 6 months post-implantation (p = 1.000). Using a Bonferroni correction, Strattice® graft-tissue composites demonstrated significant improvement in neovascularization scores at 12 months post-implantation compared to neovascularization scores at 1 month post-implantation (p = 0.0139). Strattice® graft-tissue composites approached but did not reach significant difference in neovascularization scores at 1 and 6 months post-implantation, and no significant difference was detected between neovascularization scores at 6 and 12 months post-implantation (p = 0.0202 and p = 0.1306, respectively). Strattice® graft-tissue composites exhibited significantly greater neovascularization scores compared to Permacol® graft-tissue composites at 1 and 12 months post-implantation (p = 0.0272 and p = 0.0296, respectively), although no significant differences were detected in the neovascularization scores for Permacol® and Strattice® graft-tissue composites at 6 months post-implantation (p = 0.0595).
Composite scores
Composite scores (Fig. 2g) were calculated as the mean of the six component scores (cellular infiltration, cell types, ECM deposition, scaffold degradation, fibrous encapsulation, and neovascularization) for a given specimen. The composite scores for explanted Permacol® graft-tissue composites were 1.40 ± 0.12 at 1 month post-implantation, 2.08 ± 0.08 at 6 months post-implantation, and 2.36 ± 0.12 at 12 months post-implantation. The composite scores for explanted Strattice® graft-tissue composites were 1.66 ± 0.05 at 1 month post-implantation, 2.72 ± 0.03 at 6 months post-implantation, and 2.81 ± 0.02 at 12 months post-implantation. Using a Bonferroni correction, Permacol® graft-tissue composites demonstrated significant improvement in composite scores at 6 and 12 months post-implantation compared to composite scores at 1 month post-implantation (p = 0.0023 and p = 0.0016, respectively); however, no significant difference was detected between composite scores at 12 months post-implantation compared to 6 months post-implantation (p = 0.0519). Using a Bonferroni correction, Strattice® graft-tissue composites approached but did not reach significant difference in composite scores at 6 and 12 months post-implantation compared to composite scores at 1 month post-implantation (p = 0.0209 and p = 0.0209, respectively), and no significant difference was detected between composite scores at 6 and 12 months post-implantation (p = 0.0433). No significant difference in composite scores was detected between Strattice® graft-tissue composites and Permacol® graft-tissue composites at 1 month post-implantation (p = 0.1742); however, Strattice® graft-tissue composites exhibited significantly greater composite scores compared to Permacol® graft-tissue composites at 6 months (p = 0.0066), and 12 months post-implantation (p = 0.0066).
Uniaxial tensiometry
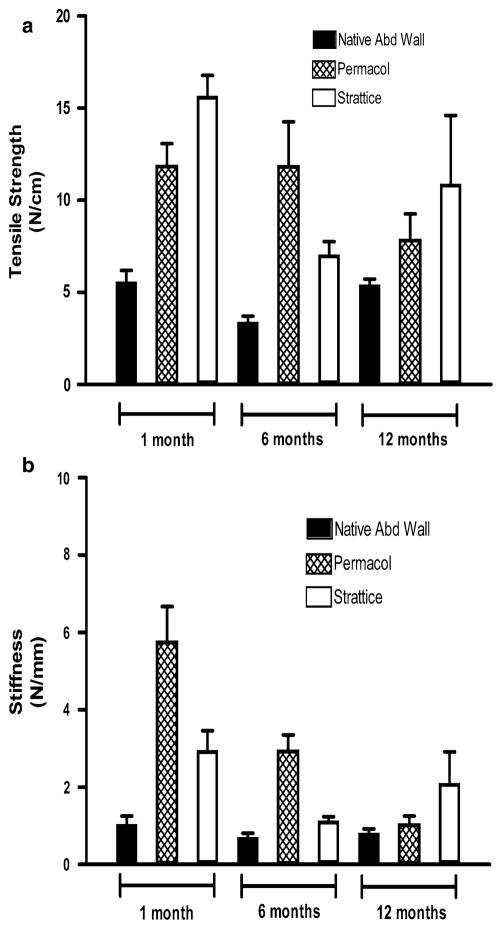
Tensile strength (N/cm)
The tensile strength per unit width (Fig. 3a) for native porcine abdominal wall was 5.59 ± 0.6, 3.40 ± 0.3, and 5.42 ± 0.3 N/cm for specimens procured at the 1 month (n = 10), 6 month (n = 15), and 12 month (n = 14) time points, respectively.
Fig. 3.
Biomechanical characteristics of mesh-repaired sites over time, compared to native porcine abdominal wall. a Tensile strength (N/cm), b stiffness (N/mm)
The tensile strength per unit width for Permacol® graft-tissue composites was 11.88 ± 1.2 N/cm at 1 month post-implantation, 11.85 ± 2.4 N/cm at 6 months post-implantation, and 7.86 ± 1.4 N/cm at 12 months post-implantation. Permacol® graft-tissue composites procured at 1 and 6 months post-implantation exhibited significantly greater tensile strength compared to native porcine abdominal wall at the respective time point (p = 0.0007 and p = 0.0012, respectively), although this was not true of Permacol® graft-tissue composites procured at 12 months post-implantation (p = 0.4128).
The tensile strength per unit width for Strattice® graft-tissue composites was 15.67 ± 1.1 N/cm at 1 month post-implantation, 7.05 ± 0.7 N/cm at 6 months post-implantation, and 10.91 ± 3.7 N/cm at 12 months post-implantation. Strattice® graft-tissue composites procured at 1 and 6 months post-implantation exhibited significantly greater tensile strength compared to native porcine abdominal wall at the respective time point (p = 0.0047 and p = 0.0027, respectively), although this was not true of Strattice® graft-tissue composites procured at 12 months post-implantation (p = 0.1371).
No statistically significantly differences were demonstrated in the tensile strength per unit width of Permacol® graft-tissue composites versus Strattice® graft-tissue composites explanted at 1, 6, or 12 months post-implantation (p = 0.0613, p = 0.0894, and p = 0.4969, respectively).
Stiffness (N/mm)
The stiffness (Fig. 3b) for native porcine abdominal wall was 1.05 ± 0.2, 0.71 ± 0.1, and 0.82 ± 0.1 N/mm for specimens procured at the 1 month (n = 10), 6 month (n = 15), and 12 month (n = 14) time points, respectively.
The stiffness for Permacol® graft-tissue composites was 5.77 ± 0.9 N/mm at 1 month post-implantation, 2.95 ± 0.4 N/mm at 6 months post-implantation, and 1.05 ± 0.2 N/ mm at 12 months post-implantation. Permacol® graft-tissue composites procured at 1 and 6 months post-implantation exhibited significantly greater stiffness compared to native porcine abdominal wall at the respective time point (p = 0.0005 and p = 0.0003, respectively), although this was not true of Permacol® graft-tissue composites procured at 12 months post-implantation (p = 0.4128).
The stiffness for Strattice® graft-tissue composites was 2.96 ± 0.5 N/mm at 1 month post-implantation, 1.14 ± 0.1 N/mm at 6 months post-implantation, and 2.11 ± 0.8 N/mm at 12 months post-implantation. Strattice® graft-tissue composites procured at 1 month post-implantation exhibited significantly greater stiffness compared to native porcine abdominal wall explanted at the respective time point (p = 0.0109); however, no significant difference in stiffness was detected between Strattice® graft-tissue composites and native abdominal wall specimens explanted at 6 or 12 months post-implantation (p = 0.0719 and p = 0.1674, respectively).
No statistically significantly differences were demonstrated in the stiffness of Permacol® graft-tissue composites versus Strattice® graft-tissue composites explanted at 1, 6, or 12 months post-implantation (p = 0.0415, p = 0.0415, and p = 0.3082, respectively).
Discussion
The ideal biologic scaffold for abdominal wall reconstruction not only optimizes pathogen clearance from contaminated and infected surgical sites, but also avoids a biologic footprint that could elicit an inflammatory immune response and fibrosis from the host, gradually degrades to allow for high-integrity host tissue regeneration, and provides an effective and durable soft tissue repair. It is widely believed that collagen crosslinking renders the biologic scaffold more resistant to degradation and augments the integrity of the soft tissue repair in vivo. However, adding bonds between collagen helices can result in an unintended foreign body response to the collagen molecules. Furthermore, biochemical processes utilized during manufacture can yield poorly controlled collagen crosslinking and may result in reduced scaffold porosity and restricted host cell infiltration of the scaffold. Ultimately, these processes may lead to fibrous encapsulation rather than tissue remodeling of the biologic scaffold for an effective soft tissue repair. Largely restricted by the proprietary nature of these manufacturing processes, independent investigation of the impact of collagen crosslinking on the histologic remodeling and biomechanical properties of the resultant scaffolds, and the clinical outcomes of their use in soft tissue repair have been limited.
The objective of the present study was to evaluate the histologic remodeling profile and biomechanical properties of the porcine abdominal wall over a 1-year period after repair with crosslinked or non-crosslinked biologic scaffolds. To achieve this end, porcine dermis allografts were selected for comparison in a porcine model of ventral hernia repair: Permacol® Surgical Implant (Covidien Surgical; Norwalk, CT; crosslinked porcine dermis scaffold) and Strattice® Reconstructive Tissue Matrix (LifeCell Corporation; Branchburg, NJ; non-crosslinked porcine dermis scaffold). It was hypothesized that hernia sites repaired with crosslinked biologic mesh would demonstrate less remodeling and greater tensile strength and stiffness than hernia sites repaired with non-crosslinked biologic mesh in a porcine model of ventral hernia repair.
The range of cellular infiltration scores observed for both Permacol® and Strattice® graft-tissue composites exhibit improvement from cellular infiltration at the scaffold periphery to cellular penetration into the scaffold center from 1 to 12 months post-implantation (Table 1). Both Permacol® and Strattice® graft-tissue composites demonstrate improvement in cell type scores from the presence of primarily inflammatory cells and scant fibroblasts at 1 month post-implantation to the near-exclusive presence of fibroblasts and absence of inflammatory cells by 12 months post-implantation. As for ECM deposition, the range of scores for Permacol® graft-tissue composites indicate improvement from host ECM deposition at the scaffold periphery at 1 month post-implantation to ECM deposition at the center of the scaffold by 6 months post-implantation with some reduction in ECM deposition by 12 months; whereas, the range of ECM deposition scores for Strattice® graft-tissue composites demonstrate improvement from host ECM deposition beyond the periphery of the scaffold at 1 month post-implantation to ECM deposition at the scaffold center by 12 months post-implantation. This represents earlier ECM deposition for hernia repair sites reinforced with the non-crosslinked porcine dermis compared to the cross-linked porcine dermis. The range of scaffold degradation scores for both Permacol® and Strattice® graft-tissue composites reveals improvement from the intact presence of the original scaffold with clear demarcation of the borders at 1 month post-implantation to extreme degradation of the original scaffold with almost complete indistinction from host tissue by 12 months post-implantation. Both Permacol® and Strattice® graft-tissue composites exhibit improvement in fibrous encapsulation scores from extensive encapsulation of the scaffold periphery at 1 month post-implantation to the absence of fibrous encapsulation by 12 months post-implantation. As for neovascularization, the range of scores for Permacol® graft-tissue composites demonstrates improvement from the presence of blood vessels at the scaffold periphery to penetration of the scaffold center by blood vessels by 12 months; whereas, the range of neovascularization scores for Strattice® graft-tissue composites demonstrates improvement from blood vessel penetration beyond the periphery of the scaffold at 1 month post-implantation to blood vessel penetration into the scaffold center by 12 months post-implantation. This suggests earlier neovascularization of hernia repair sites reinforced with the non-crosslinked porcine dermis compared to the crosslinked porcine dermis.
Given analogous findings reported for crosslinked and non-crosslinked bovine pericardium graft-tissue composites in this model [19], it was expected that both crosslinked and non-crosslinked porcine dermis graft-tissue composites would demonstrate more evidence of remodeling with greater duration of time in vivo. Permacol® graft-tissue composites demonstrated statistically significant improvement in cellular infiltration, ECM deposition, neovascularization, and composite remodeling at 6 and 12 months compared to 1 month post-implantation, and statistically significant improvement in cell type and fibrous encapsulation at 12 months compared to 1 month post-implantation; however, a significant difference between 6 and 12 months post-implantation was only detected for ECM deposition and fibrous encapsulation for Permacol® graft-tissue composites. Strattice® graft-tissue composites demonstrated statistically significant improvement in fibrous encapsulation at 6 and 12 months compared to 1 month post-implantation, and in neovascularization at 12 months compared to 1 month post-implantation. Strattice® graft-tissue composites approached but did not reach significant difference in cell type, ECM deposition, scaffold degradation, and composite remodeling at 6 and 12 months compared to 1 month post-implantation, and in neovascularization at 6 months compared to 1 month post-implantation. No significant differences between 6 and 12 months post-implantation were detected for any category of the remodeling scale for Strattice® graft-tissue composites. Thus, the crosslinked graft-tissue composites exhibited statistically significant improvement in a greater number of remodeling characteristics over the study period than the non-crosslinked graft-tissue composites.
As the antithesis of what one might expect for a cross-linked scaffold and a non-crosslinked scaffold, respectively, Permacol® graft-tissue composites demonstrated significant improvement over time in every remodeling category except scaffold degradation, while the remodeling characteristics of Strattice® graft-tissue composites remained relatively unchanged over time for every category except fibrous encapsulation and neovascularization which demonstrated improvement. However, it should be noted that scores for the Strattice® graft-tissue composites were already significantly higher after just 1 month compared to the Permacol® graft-tissue composites in the remodeling categories of cellular infiltration, ECM deposition, and neovascularization, providing evidence of earlier remodeling of the non-crosslinked grafts compared to the crosslinked grafts. By 12 months, ECM deposition, scaffold degradation, neovascularization, and overall composite remodeling scores were significantly higher for the Strattice® graft-tissue composites compared to the Permacol® graft-tissue composites. Scores for cellular infiltration, cell types, and fibrous encapsulation eventually reached similar levels at 12 months for both grafts regardless of crosslinking. Furthermore, ECM deposition and neovascularization levels started out higher at 1 month and remained higher at 12 months for the Strattice® graft-tissue composites compared to the Permacol® graft-tissue composites. Thus, while the crosslinked graft-tissue composites exhibited statistically significant improvement in a greater number of remodeling characteristics over the study period than the non-crosslinked graft-tissue composites, the non-crosslinked graft-tissue composites still demonstrated a greater degree of remodeling overall at the 6 and 12 month time points compared to the crosslinked graft-tissue composites. It is postulated that these histologic findings may correlate with more durable hernia repairs at earlier time points with the use of non-crosslinked porcine dermis compared to HDMI-crosslinked porcine dermis; however, the translation to improved clinical outcomes remains to be proven in future clinical studies.
Both crosslinked and non-crosslinked graft-tissue composites exhibited significantly greater tensile strength than the native abdominal wall without mesh at the early time points. That is, the tensile strength of both crosslinked and non-crosslinked graft-tissue composites remained greater than the tensile strength of the native porcine abdominal wall up to 6 months post-implantation. However, there was no difference in the tensile strength of the graft-repaired sites and the native abdominal wall at 12 months post-implantation. This implies that biologic scaffold reinforcement does augment the uniaxial tensile strength of abdominal wall repair sites compared to the native abdominal wall tensile strength in the early post-operative period in a porcine model. Moreover, it was expected that crosslinked graft-tissue composites would exhibit significantly greater tensile strength compared to non-crosslinked graft-tissue composites at 1, 6, and 12 months post-implantation. However, no significant difference was detected in the tensile strength of Permacol® versus Strattice® graft-tissue composites at 1, 6, and 12 months post-implantation. Taken together, these data suggest that while porcine dermis scaffold reinforcement does augment the uniaxial tensile strength of the abdominal wall repair site compared to native abdominal wall in the early post-operative period, there is no added benefit to the tensile strength of graft-tissue composites when crosslinking porcine dermis with HDMI compared to the native abdominal wall, as assessed up to 12 months post-implantation in a porcine model of ventral hernia repair. Further studies are warranted to compare the uniaxial tensile strength of abdominal wall repair sites with biologic scaffold reinforcement versus abdominal wall sites with primary suture repair alone.
Both crosslinked and non-crosslinked graft-tissue composites exhibited significantly greater stiffness than the native abdominal wall without mesh at the earliest time point. That is, the stiffness of both crosslinked and non-crosslinked graft-tissue composites was greater than the stiffness of the native porcine abdominal wall at 1 month post-implantation. However, only the crosslinked graft-tissue composites demonstrated significantly greater stiffness than the native porcine abdominal wall at 6 months post-implantation. Neither graft demonstrated significantly different stiffness compared to the native porcine abdominal wall at 12 months post-implantation. This implies that biologic scaffold reinforcement does augment the stiffness of the abdominal wall repair sites compared to the native abdominal wall stiffness in the very early post-operative period in a porcine model, but that these effects do not persist at longer time points. Moreover, it was expected that crosslinked graft-tissue composites would exhibit significantly greater stiffness compared to non-crosslinked graft-tissue composites at 1, 6, and 12 months post-implantation. However, no significant differences were noted between the stiffness of Permacol® versus Strattice® graft-tissue composites at 1, 6, and 12 months post-implantation. Taken together, these data suggest that while porcine dermis scaffold reinforcement does augment the stiffness of the abdominal wall repair site compared to native abdominal wall in the very early post-operative period in a porcine model, there is no added benefit to the stiffness of graft-tissue composites when crosslinking porcine dermis with HDMI compared to the native abdominal wall, as assessed up to 12 months post-implantation in a porcine model of ventral hernia repair.
The results of this experimental study must be interpreted in light of its inherent limitations. This study is a preclinical trial utilizing a porcine model of open ventral hernia repair with preperitoneal retromuscular biologic scaffold reinforcement. The ventral hernia defects did not naturally occur in the pigs, but rather were created by incision and matured over 21 days before open repair in this model. These hernia defects were created lateral to the midline to accommodate two repair sites per animal; therefore, the model may not recapitulate the abdominal wall forces experienced with a midline ventral hernia defect, Since Permacol® and Strattice® scaffolds were not implanted in the same animal, inherent differences in the host pigs may have contributed to some variation in the outcomes. While the porcine abdominal wall closely approximates the human abdominal wall in structure and function, allograft scaffold remodeling following open ventral hernia repair in the porcine model may approximate but not fully represent xenograft scaffold remodeling following open ventral hernia repair in humans. As is true of biologic scaffold reinforcement of any soft tissue repair, the tissue attributes of the donor species and the individual donor, and the processing techniques of the commercial manufacturer may also cause outcomes to vary with each individual scaffold application. Moreover, the results of this study apply only to the Permacol® and Strattice® porcine dermis scaffolds utilized. The authors caution against generalizations of the study results to other brands of crosslinked and non-crosslinked porcine dermis scaffolds, other ventral hernia repair techniques, and other clinical conditions such as host comorbidity burden or surgical site contamination. Tensiometric analyses of the graft-tissue composites were performed on specimens trimmed to a single muscle layer of thickness to accommodate the 1.5 cm aperture of the Instron Series 5542 Universal Testing System pneumatic grips. This may have resulted in modest to negligible underestimations of the tensile strength and stiffness of the composite specimens.
Despite limited sample sizes, several significant differences were discovered in this analysis between HDMI-crosslinked porcine dermis and non-crosslinked porcine dermis reinforcement of porcine ventral hernia repair sites. It should be noted, however, that the absence of other significant differences does not necessarily imply that these differences do not exist; rather, the study may not have been sufficiently powered to detect them. Assuming that the results are not significantly impacted by the inherent characteristics of the porcine species and individual graft donors, and the proprietary manufacturing processes, the data indicate that collagen crosslinking with HDMI significantly reduces the histologic remodeling profile and does not significantly impact the tensile strength or stiffness of the graft-tissue composites in a porcine model of ventral hernia repair up to 12 months post-implantation. It should be noted, however, that Strattice® scaffolds may possess an unquantified degree of unintentional collagen crosslinking as a result of the radiation used during sterilization and the dehydration process used for long-term preservation. Until transparency is achieved, the proprietary nature of these manufacturing processes may preclude more accurate analysis of the impact of unintended collagen crosslinking on the histologic and biomechanical properties of biologic scaffolds.
Acknowledgments
This research was supported by a grant from Synovis Surgical Innovations, Inc., St. Paul, MN. This data were presented at Hernia Repair 2011, American Hernia Society, San Francisco, California, March 18, 2011. Funding for this project was provided by Synovis Surgical Innovations, Inc. (St. Paul, MN).
Footnotes
Presented at Hernia Repair 2011, American Hernia Society, San Francisco, California, March 18, 2011.
Conflict of interest B.D.M. declares having received funds in relation with this study.
Contributor Information
J. A. Cavallo, Department of Surgery, Section of Minimally Invasive Surgery, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8109, St. Louis, MO 63110, USA
S. C. Greco, Division of Comparative Medicine, Washington University School of Medicine, St. Louis, MO, USA
J. Liu, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO, USA
M. M. Frisella, Department of Surgery, Section of Minimally Invasive Surgery, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8109, St. Louis, MO 63110, USA
C. R. Deeken, Email: deekenc@wudosis.wustl.edu, Department of Surgery, Section of Minimally Invasive Surgery, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8109, St. Louis, MO 63110, USA
B. D. Matthews, Department of Surgery, Section of Minimally Invasive Surgery, Washington University School of Medicine, 660 South Euclid Avenue, Campus Box 8109, St. Louis, MO 63110, USA
References
- 1.Millennium Research Group. US markets for soft tissue repair devices. Millennium Research Group Incorporated; Toronto: 2010. [Google Scholar]
- 2.Harth KC, Broome AM, Jacobs MR, Blatnik JA, Zeinali F, Bajaksouzian S, Rosen MJ. Bacterial clearance of biologic grafts used in hernia repair: an experimental study. Surg Endosc. 2011;25(7):2224–2229. doi: 10.1007/s00464-010-1534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006;192(6):705–709. doi: 10.1016/j.amjsurg.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Patton JH, Jr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg. 2007;193(3):360–363. doi: 10.1016/j.amjsurg.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Maurice SM, Skeete DA. Use of human acellular dermal matrix for abdominal wall reconstructions. Am J Surg. 2009;197(1):35–42. doi: 10.1016/j.amjsurg.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26(4):507–523. doi: 10.1016/j.cpm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28(25):3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Badylak SF. Xenogeneic extracellular matric as a scaffold for tissue reconstruction. Transpl Immunol. 2004;23(3–4):367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res. 2000;32(1):43–48. doi: 10.1159/000008740. [DOI] [PubMed] [Google Scholar]
- 11.Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of crosslinking degree of an acellular biologic tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541–3552. doi: 10.1016/j.biomaterials.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Care and Use of Laboratory Animals, U.S. Department of Health and Human Services, Public Health Service. National Institutes of Health; Bethesda, MD: 1996. [Google Scholar]
- 13.de Castro Bras LE, Proffitt JL, Bloor S, Sibbons PD. Effect of crosslinking on the performance of collagen-derived biomaterial as an implant for soft tissue repair: a rodent model. J Biomed Mater Res B Appl Biomater. 2010;95(2):239–249. doi: 10.1002/jbm.b.31704. [DOI] [PubMed] [Google Scholar]
- 14.International consensus. An expert working group review. Wounds International; London: 2010. Acellular matrices for the treatment of wounds. [Google Scholar]
- 15.Permacol® Surgical Implant: Instructions for Use. Tissue Science Laboratories, Incorporated; Andover: 2007. www.tissuescience.com. [Google Scholar]
- 16.Strattice® Reconstrutive Tissue Matrix: Instructions for Use. LifeCell Corporation; Branchburg: 2008. http://www.lifecell.com/downloads/StratticeIFU_T11.pdf. [Google Scholar]
- 17.Jenkins ED, Melman L, Desai S, Brown SR, Frisella MM, Deeken CR, Matthews BD. Evaluation of intraperitoneal placement of absorbable and nonabsorbable barrier coated mesh secured with fibrin sealant in a New Zealand white rabbit model. Surg Endosc. 2011;25(2):604–612. doi: 10.1007/s00464-010-1230-8. [DOI] [PubMed] [Google Scholar]
- 18.Melman L, Jenkins ED, Hamilton NA, Bender LC, Brodt MD, Deeken CR, Greco SC, Frisella MM, Matthews BD. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia. 2011;15(2):157–164. doi: 10.1007/s10029-010-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeken CR, Melman L, Jenkins ED, Greco SC, Frisella MM, Matthews BD. Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg. 2011;212(5):880–888. doi: 10.1016/j.jamcollsurg.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]