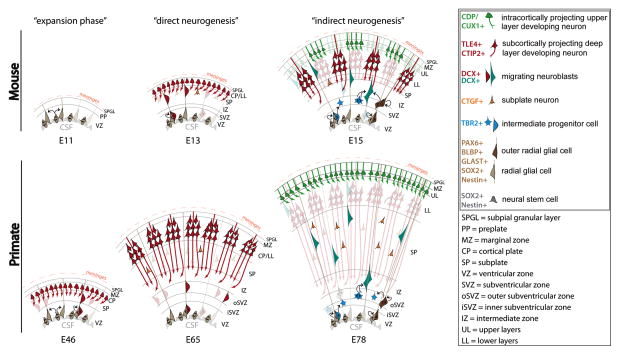
Figure 2. Schematic of distinct stages of neocortical neurogenesis in developing mouse and primate neocortices.
The first “phase” of neocorticogenesis in mouse and primates is characterized by symmetric divisions of neural stem cells called neuroepithelium cells (NEC), which amplifies the number of neocortical progenitors at the ventricular zone (VZ) surface (left panels). This initial phase is accordingly called the “expansion phase”. NECs will then transition into a different lineage of neocortical neural stem cells called radial glia (RG), which divide asymmetrically and first predominantly produce neuronal progeny. This phase was named “direct neurogenesis” (middle panels). As the neurogenic phase progresses, RG continue to undergo a series of asymmetric divisions, but they predominantly produce another progenitor subtype, intermediate progenitor cells (IPCs) and outer radial glia (oRG) (right panels). IPCs and oRG will divide in the subventricular zone (SVZ), which in primates is divided into inner (iSVZ) and outer (oSVZ) portions. Importantly, IPCs terminally divide symmetrically and produce at least two neural progenies. However, oRG will self renew and give rise to neural progenies and IPCs. In this way, both IPCs and oRGs amplify the output of RG, and thus, this later stage of neurogenesis was named “indirect neurogenesis”. These progressive changes in differentiation of neocortical neural stem cells define the birth of distinct subpopulations of projection neurons. Deep layer neurons that will project subcortically into thalamus, brain stem and spinal cord will be born before upper layer intracortically projecting neurons. (Adapted from Angevine and Sidman, 1961, Rakic, 1974, Smart et al., 2002, and Molnár et al., 2006).