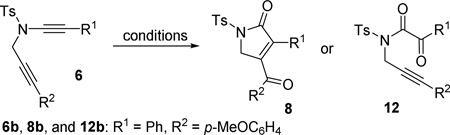
Table 1.
Screening of Conditions for the Oxidative Cycloisomerization of Diyne 6a
 | |||
|---|---|---|---|
| Entry | Conditions | 8b / 12bb | Yieldc |
| 1 | [Rh(CO)2Cl]2 (5 mol%), P[OCH(CF3)2]3 (20 mol%) | 1 : 0 | 62% |
| 2 | [Rh(CO)2Cl]2 (5 mol%) | 5 : 1 | - |
| 3 | [Rh(CO)2Cl]2 (5 mol%), P(OPh)3 (20 mol%) | no reaction | |
| 4 | [Rh(CO)2Cl]2 (5 mol%), [3,5-(CF3)2C6H3]3P (20 mol%) | 5 : 1 | - |
| 5 | Rh2(OAc)4 (5 mol%) | no reaction | |
| 6 | Au(PPh3)Cl (10 mol%), AgOTf (10 mol%) | 0 : 1 | - |
| 7 | PtCl2 (5 mol%) | complex mixture | |
| 8d | See entry 1 for catalyst | 1 : 0 | 70% |
| 9d,e | See entry 1 for catalyst | 1 : 0 | 78% |
Conditions: pyridine N-oxide (3 equiv), ClCH2CH2Cl, 80 °C, 4h, unless noted otherwise.
The ratio was determined by 1H NMR of crude product.
Isolated yield of 8b.
3,5-dichloropyridine N-oxide (3 equiv) was used as the oxidant.
Dioxane was used as the solvent.
