Abstract
Parenteral nutrition (PN) is a life-saving nutritional support for a large population of hospitalized infants, and lipids make a substantial contribution to their energy and essential fatty acid (FA) needs. A challenge in the care of these infants is that their metabolic needs require prolonged PN support that increases the risk of PN-associated liver disease (PNALD). In recent years, the emergence of new parenteral lipid emulsions containing different source lipids and FA profiles has created nutritional alternatives to the first-generation, soybean oil–based lipid emulsion Intralipid. The limited U.S. introduction of the new-generation fish-oil emulsion Omegaven has generated promising results in infants with PNALD and spawned a renewed interest in how PN and lipid emulsions, in particular, contribute to this disease. Studies suggest that the lipid load and constituents, such as specific FAs, ratio of n–3 (ω-3) to n–6 (ω-6) long-chain polyunsaturated FAs, phytosterols, and vitamin E content, may be involved. There is an existing literature describing the molecular mechanisms whereby these specific nutrients affect hepatic metabolism and function via lipid and bile acid sensing nuclear receptors, such as peroxisome proliferator–activated receptor α, liver X receptor, and farnesoid X receptor, yet virtually no information as to how they interact and modulate liver function in the context of PN in pediatric patients or animal models. This article will review the recent development of parenteral lipid emulsions and their influence on PNALD and highlight some of the emerging molecular mechanisms that may explain the effects on liver function and disease.
Introduction
Parenteral nutrition (PN)7 is a life-saving nutritional support for more than half a million premature and low-birth-weight infants and other hospitalized infants in the United States annually. The immaturity and dysfunction of their gastrointestinal tract contributes to increased morbidity, and thus many premature infants receive PN to fulfill their nutritional needs. A recent analysis of all infants admitted to the neonatal intensive-care units in a multicenter health care network found that 79% received PN for up to 2 wk, and 15% got PN for up to 1 mo (1). Importantly, those infants that were smaller and more premature received longer duration of PN support. A challenge in the care of premature infants is to provide sufficient nutrition to meet their high metabolic needs for growth, but aggressive administration of PN increases the risk of metabolic liver disease (2, 3).
Cholestatic liver disease is one of the most common metabolic problems associated with PN in premature infants. In infants that receive PN for at least 2 mo, the incidence of cholestatic liver disease may be as high as 50% and can eventually lead to end-stage liver disease and need for transplant (4). Cholestatic liver disease, a key element of PN-associated liver disease (PNALD) in infants presents clinically as increased serum biochemical markers, such as bilirubin, γ-glutamyl transpeptidase, bile acids, and liver transaminases (1, 4), and, in many patients, steatosis may occur, but this often goes undetected because of the risk of a liver biopsy (5). The risk factors associated with PN-associated cholestasis include developmental immaturity in the hepatic transport and metabolism of bile acids, lack of enteral feeding, and infection, including sepsis. Despite these known risk factors, the etiology of PN-associated cholestasis and liver disease in infants is not established, and currently there are no proven effective therapeutic treatments. The goal of this review is to examine recent reports demonstrating beneficial effects of new-generation lipid emulsions and discuss several emerging cellular and molecular mechanisms that could explain how the composition of parenteral lipids affect hepatic metabolism, function, and disease susceptibility.
New-Generation Lipid Emulsions
Lipids make a significant contribution to the energy and essential FA needs of parenterally fed infants. In the United States, the primary FDA-approved lipid emulsion is Intralipid, a soybean-oil emulsion, along with Liposyn II, a 50%:50% soybean oil/safflower oil blend. Intralipid is enriched with the FAs linoleic acid (n–6, 53%) and oleic acid (n–9, 24%) but is devoid of DHA and EPA (n–3) long-chain PUFA (LC-PUFA). Intralipid also contains phytosterols, which are steroid compounds with cholesterol-like structure occurring naturally in vegetable oils and their products. Several newer parenteral lipid emulsions have been developed in the past 10–15 y containing single-source lipid or blends of lipids (6); these more recently developed lipid emulsions have been termed “new generation.” New-generation parenteral lipid emulsions containing pure olive oil (Clinoleic), pure fish oil (Omegaven), or various blends of soy, olive, medium-chain TGs, and fish oil (Lipofundin, SMOFlipid, Lipoplus) have been approved in Europe (Table 1).
TABLE 1.
Lipid sources and proportions for Intralipid and new-generation emulsions
| Product | Soybean | Safflower | Olive | Medium-Chain TGs | Fish |
| % | |||||
| Intralipid1 | 100 | 50 | |||
| Liposyn II2 | 50 | ||||
| Omegaven1 | 100 | ||||
| SMOFlipid1 | 30 | 25 | 30 | 15 | |
| Lipoplus3 | 40 | 50 | 10 | ||
| Lipofundin3 | 50 | 50 | |||
| Clinoleic4 | 20 | 80 | |||
Fresenius Kabi.
Hospira.
B. Braun.
Baxter Corporation.
Recent clinical studies in infants show that parenteral soybean-oil lipid emulsions are linked to cholestasis and that reducing the lipid load gives reduced cholestasis; this strategy has been referred to as lipid minimization (7, 8). Omegaven is currently under FDA review but is approved under a restricted, compassionate-use protocol for administration of no more than 1 g · kg−1 · d−1 lipid to infants with cholestasis. Several pediatric centers are providing this to children who develop PNALD (9–13). Omegaven is enriched in n–3 LC-PUFA (DHA and EPA) and vitamin E but low in linoleic acid and devoid of phytosterols compared with Intralipid. Recent positive reports from small pediatric trials of PN-fed short-bowel patients have shown that infants that were given previously 1–3 g · kg−1 · d−1 Intralipid experienced a reduction in cholestasis and triglyceridemia after they were switched to 1 g · kg−1 · d−1 Omegaven infusion (10, 11, 13). Most previous clinical studies did not control for the lipid load between Intralipid and Omegaven groups, and thus it remains unknown whether the reduction in lipid load or type of lipid emulsion explains the metabolic benefit. Furthermore, there are no established mechanisms that would explain why either lipid load or FA composition confers the optimal metabolic function and prevention of PNALD.
Parenteral n–3 LC-PUFA Metabolism in Infants
There is a large body of evidence showing that fish oil and its constituent FAs have numerous biologic actions that result in improved metabolic health and reduced risk of diseases, especially those associated with inflammation, such as ischemic heart disease (14, 15). This has prompted a recent push for the development of dietary recommendations for intake of specific FAs that are enriched in fish oil (16). The beneficial health effects of fish oil are believed to be mediated by the key bioactive n–3 LC-PUFA, namely DHA and EPA. Human breast milk contains DHA and EPA, and, in recent years, commercial infant formulas have been reformulated to contain DHA and arachidonic acid (n–6 LC-PUFA), mainly to support neurodevelopment.
The influence of n–3 LC-PUFA when given parenterally on the health, metabolic function, and development of human infants is essentially unknown at present but is rapidly becoming a topic of interest in pediatric nutrition. As mentioned previously, most parenterally fed infants in the United States are given the soybean oil–based lipid emulsion (Intralipid), which is devoid of DHA and EPA (n–3) LC-PUFA. However, given the recent positive reports from small pediatric trials of PN-fed short-bowel patients, there is considerable interest in the fish oil–based lipid emulsion Omegaven (10, 11, 13). Omegaven is enriched in the n–3 LC-PUFAs DHA and EPA but low in the n–6 LC-PUFA linoleic acid. An emerging consensus based on studies in adults and recently in premature infants suggests that increasing the ratio of dietary n–3 to n–6 LC-PUFA promotes health and metabolic function (17). Despite this evidence, there is a wide range in the doses of n–3 LC-PUFA administered in adult supplementation, infant formula studies, and the low-dose, parenteral Omegaven as monotherapy trials. Thus, there is a compelling rationale to establish whether the beneficial metabolic effects of n–3 LC-PUFA occur in parenterally fed infants and what dose ranges are safe and effective.
Role of n–3 LC-PUFA on Metabolic Function and PNALD
Fish oil has emerged as a dietary supplement to ameliorate the metabolic dysfunction associated with symptoms of obesity, such as increased serum lipids, glucose, insulin resistance, and steatosis. Studies in obese adult humans and rodents show that dietary fish-oil supplements increase insulin sensitivity, reduce serum lipids, and also appears to reduce serum liver transaminases and histologic signs of steatohepatitis (18, 19). One of the important mechanisms by which n–3 LC-PUFA, especially DHA and EPA, affect metabolic function is via changing the expression of genes involved in fat and glucose metabolism (20, 21). Their action suppresses the transcription of genes encoding transcription factors, such as sterol regulatory element binding protein and carbohydrate-responsive element-binding protein (20) and specific lipogenic enzymes and induces the expression of genes encoding specific enzymes involved in peroxisomal and microsomal FA oxidation. The net effect of n–3 LC-PUFA on gene expression is to reduce lipid accumulation in the liver. The beneficial metabolic effects of n–3 LC-PUFA or the resultant change in the balance of the ratio of n–3 to n–6 LC-PUFA have also been linked to other cellular mechanisms, including suppression of inflammation via mechanisms involving eicosanoids metabolism and/or production of resolvins E and D (22, 23).
At the cellular level, the actions of these bioactive FAs are thought to be mediated by their uptake via FA transport protein and binding to FA binding protein, which acts as a chaperone to facilitate molecular binding and activation of nuclear receptors, specifically peroxisome proliferator-activated receptor (PPAR) α/γ (24, 25). The transcriptional induction of PPARα-responsive genes promotes hepatic intracellular FA uptake, the conversion of FAs to their acyl-CoA derivatives, and channeling toward mitochondrial/peroxisomal oxidation (19, 26). The hepatic expression of PPARα is significantly reduced in mice fed a high-fat diet (18). Thus, the presence of PPARα or its activation by fish-oil supplementation has been found to be protective against diet-induced obesity in mice. Fish oil–mediated activation of PPARα induces peroxisome proliferation and upregulation of genes involved in mitochondrial and peroxisomal FA oxidation, leading to partitioning of lipid metabolites toward degradation and reduced accumulation in the liver. Furthermore, PPARα acts to reduce serum TG concentrations by transcriptional regulation of high- and very-low-density apolipoproteins, resulting in increased lipolysis and clearance of remnant particles (27, 28). Studies in mice also showed that fish oil prevents fat-induced hepatic insulin resistance in vivo in a PPARα-dependent manner (18). PPARα regulates hepatic glucose metabolism via both direct effects of glycolytic and gluconeogenic pathways and indirect effects on insulin signaling (28).
Parenteral Lipid Nutrition and Hepatic Lipotoxicity
PNALD is marked by a metabolic phenotype with many similarities to nonalcoholic fatty liver disease (NAFLD) evident as hepatic lipid accumulation, steatosis, tissue injury, as well as insulin resistance and inflammation (4, 5, 29–32). NAFLD is the most common cause of chronic liver disease in children and adolescents and has been listed as a comorbidity of obesity (33, 34). One of the most severe forms of NAFLD is nonalcoholic steatohepatitis. The pathobiology of nonalcoholic steatohepatitis has been described by the “two-hit” hypothesis, in which, first, relatively benign hepatic lipid accumulation occurs but is followed by the second hit that is an inflammatory insult that triggers tissue injury and fibrosis. In the context of obesity, the process of hepatocyte lipid accumulation occurs as a result of the increased circulating load of FFAs released from adipose tissue. During steatosis, it is the FFAs rather than TGs that are inherently toxic to liver cells (35). Moreover, studies have demonstrated that saturated FAs, especially palmitate, induced hepatocyte lipid accumulation and are more toxic than unsaturated FAs (31, 36–38). Treatment of cultured cells, including hepatocytes, with palmitate induces lipotoxicity resulting from oxidant stress attributable to overproduction of mitochondrial reactive oxygen species (ROS) and endoplasmic reticulum stress (36, 39, 40). Hepatocyte lipotoxicity induced by treatment with palmitate triggers c-Jun N-terminal kinase signaling, nuclear factor κB activation, proinflammatory cytokine production, and ultimately apoptotic cell death (37, 41, 42). One of the metabolic phenotypes found in NAFLD in the context of obesity is insulin resistance, yet there is limited clinical evidence for insulin resistance associated with PNALD. Our recent studies in neonatal pigs show that, after only 2 wk of total PN (TPN) vs. enteral nutrition, there is a striking increase in insulin resistance associated with hepatic steatosis, cholestasis, and inflammation (43, 44). The induction of insulin resistance in TPN-fed piglets was associated with increased hepatic c-Jun N-terminal kinase signaling and diminished intracellular insulin signaling.
Mechanisms to explain why saturated vs. unsaturated FAs are more hepatotoxic have been linked to the activation of FA oxidation pathways via nuclear receptors, such as PPARα (24, 25). The transcriptional induction of PPARα-responsive genes promotes hepatic intracellular FA uptake and mitochondrial/peroxisomal oxidation (19, 26). Fish oil–mediated activation of PPARα induces peroxisome proliferation and upregulation of genes involved in mitochondrial and peroxisomal FA oxidation and reduced accumulation in the liver. Thus, similar to obesity, parenteral lipid infusion leads to continuous exposure of liver cells to high concentrations of FAs, which can induce steatosis, oxidative stress, and lipotoxicity. Given the similarities between the metabolic phenotypes observed in PNALD and NAFLD, we hypothesize that there are common underlying mechanisms that explain the tissue injury and dysfunction observed with these diseases.
FA Composition and Hepatic Inflammation: Role of Toll-Like Receptor 4
Inflammatory stress is a common comorbidity found in PN-fed infants attributable to the high incidence of bacterial sepsis that originates from either central-line catheters or intestinal translocation (45, 46). An unfortunate and well-established complication of sepsis-induced inflammation is cholestasis (47–51). Mechanistic studies (48–50) in animals and hepatocytes show that treatment with lipopolysaccharide from gram-negative bacteria reduces the expression of hepatic farnesoid X receptor (FXR) and bile salt export pumps (BSEPs), leading to hepatocyte bile accumulation, yet bile acid activation of FXR suppresses inflammation via nuclear factor κB (52, 53). In addition to cases of central catheter–derived bacterial sepsis, studies indicate that intestinal absorption of bacterial-derived endotoxin in conditions of PN is linked to hepatic steatosis, cholestasis, and tissue injury (43, 47). Our previous studies in neonatal pigs confirm that TPN erodes the intestinal barrier and increases permeability to luminal bacterial toxins (54). Additional evidence to support this idea comes from studies showing that metronidazole treatment inhibited intestinal bacterial overgrowth and reduced PN-associated steatosis and signs of cholestasis (55, 56). Moreover, the stimulation of inflammation by bacterial endotoxin is mediated by lipopolysaccharide activation of the toll-like receptor 4 (TLR4). Recent studies in a mouse model confirm the idea that PN-induced liver injury is associated with gut translocation of bacterial endotoxin and is dependent on activation of TRL4 in Kupffer cells (57). In addition, saturated FAs, especially palmitate, can also activate cellular inflammation not only as a trigger of lipotoxicity but also act as a ligand for TLR4 (58, 59). More importantly, this group has shown that n–3 LC-PUFA, especially DHA, inhibit palmitate-induced TLR4-activated inflammation. Thus, the absence of DHA in soy- and safflower-based lipid emulsions and DHA enrichment in fish oil–containing lipid emulsions could alter the balance between hepatic inflammation pathways and metabolic function during PN.
FXR Function and Bile Acid Homeostasis: Role of Phytosterols
Recent reports that Omegaven substantially reduces the elevated concentrations of serum markers of cholestasis in children suggest a role for FXR (9, 12). FXR is the primary bile acids sensor, and it activates the expression of short heterodimer partner. Short heterodimer partner binds to and inactivates liver receptor homolog 1, thus potently inhibiting the expression of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid synthesis. In general, FXR maintains hepatocyte bile acid homeostasis by regulating the expression of genes involved in synthesis (CYP7A1), uptake (Na+-taurocholate cotransporter polypeptide and organic anion-transporting peptide 2/8), and export of bile acid (BSEP and multidrug resistance protein 2). One mechanism implicated in PN-induced cholestatic liver disease is that plant phytosterols in soybean-oil emulsions disrupt bile acid homeostasis (60, 61). The principal phytosterols present in soybean-oil emulsions are β-sitosterol, the most abundant, and campesterol and stigmasterol. Numerous studies have reported evidence of phytosterolemia in PN-fed patients, and some show that they correlate positively with bilirubin concentrations and poor liver function tests (60, 62–66). A key study in cultured hepatocytes revealed a potential molecular link in which stigmasterol antagonized the bile acid–dependent activation of FXR target genes (67). Studies that demonstrate the direct effects of different lipid emulsions and phytosterols on hepatic bile acid synthesis and bile acid transport in vivo are emerging. Our recent studies in a preterm piglet model of PNALD compared 2 new-generation lipid emulsions (Omegaven and SMOFlipid) with Intralipid. The results show that both Omegaven and SMOFlipid prevented the development of hepatic cholestasis and steatosis that occur in piglets given Intralipid. Importantly, the hepatoprotective effect of new-generation emulsions was associated with reduced phytosterolemia (H. Vlaardingerbroek, K. Ng, B. Stoll, N. Benight, S. Chacko, L.A.J. Kluijtmans, W. Kulik, E.J. Squires, O. Olutoye, M.L. Finegold, J.B. van Goudoever, D.G. Burrin, unpublished results). This was despite the fact that SMOFlipid contains phytosterols derived from the soy and olive oils, albeit at 40% of the concentration compared with Intralipid. We also showed that phytosterols antagonize bile acid–induced FXR target gene (BSEP) expression in cultured primary piglet hepatocytes. A recent report in a mouse model showed direct evidence that plant sterols, specifically stigmasterol, in lipid emulsions are a key factor responsible for PNALD. They showed that adding stigmasterol to Omegaven induced a similar liver injury to that of Intralipid in mice given a combination of gut injury and TPN (68). Interestingly, this work implicates phytosterol activation of Kupffer cells and cytokine-mediated downregulation of hepatocyte phytosterol transporters, leading to intracellular accumulation. Together, these findings imply that the hepatoprotective responses reported with lipid minimization strategies and pure fish-oil emulsion (i.e., Omegaven) therapy may be linked to the presence or absence of phytosterols that act to induce inflammation and antagonize hepatic FXR function in bile acid homeostasis.
FXR–Fibroblast Growth Factor 19 Signaling: A Novel Enterokine Mechanism in PNALD
Studies in mice showed that FXR stimulates the transcription of fibroblast growth factor 15 (FGF15) and its human ortholog FGF19 (69, 70). Before the discovery of FXR, it was known that intestinal administration of bile acids suppresses hepatic CYP7A1, implicating a secreted intestinal factor that acts to suppress bile acid synthesis. The first evidence that FGF19 was the secreted factor came from studies showing that FGF19 repressed CYP7A1 expression in both isolated hepatocytes and mice. Subsequently, the studies by Inagaki et al. (70) showed that tissue-specific FXR knockout in either the liver or intestine increases the bile acid pool size. In addition, treatment with GW4064 (FXR-selective agonist) significantly repressed CYP7A1 in liver-specific FXR knockout mice but not in intestine-specific FXR knockout mice. This suggests that CYP7A1 repression is mediated primarily by FXR activation in the intestine and not in the liver. More recent evidence confirms this by showing that selective activation of intestinal FGF15 in mice protects against hepatic cholestasis (71). The induction of intestinal FGF19 secretion via FXR is thought to occur primarily in epithelial cells in the distal region of the ileum because this is where the bile acid transporters are most highly expressed (72). The tissue-specific localization and molecular regulation of the FXR–FGF19 axis in intestinal epithelial cells are poorly understood. The receptor for FGF15/19 is FGF receptor 4 (FGFR4), which is abundant in the liver, and mice lacking FGFR4 have an increased bile acid pool. Activation of FGF19 signaling in cells via FGFR4 also requires the coreceptor β-Klotho, and tissue-specific expression of this coreceptor is an important determinant of FGF19 responsiveness (69).
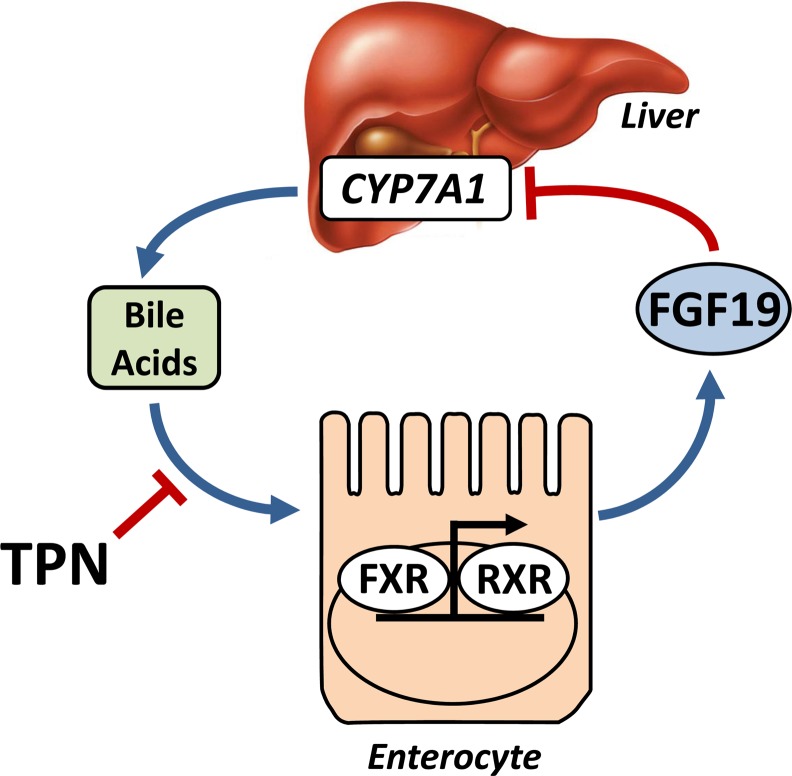
The importance of FGF19 in human bile metabolism has been shown in individuals treated with cholestyramine and the FXR ligand chenodeoxycholic acid (CDCA). Treatment with cholestyramine led to an increase in serum C4 (marker for CYP7A1 activity) and a reduction in FGF19 concentrations whereas CDCA treatment increased plasma FGF19 and decreased serum C4 (73). Our recent work demonstrated the importance of luminal bile acid stimulation for maintenance of circulating FGF19 secretion in TPN-fed neonatal pigs (44). We showed that TPN markedly decreased concentrations of circulating FGF19, whereas duodenal infusion of CDCA significantly induced FGF19. We also found that liver pathology associated with TPN was markedly improved with CDCA infusion. The finding that TPN results in reduced FGF19 secretion is novel and may provide a mechanism to explain the cholestasis and steatosis observed with PNALD. Because FGF19 production in the small intestine causes suppression of CYP7A1 in hepatocytes, diminished FGF19 concentrations in PN-fed patients could increase CYP7A1 expression in the liver, resulting in persistent activation of bile acid synthesis and further resulting in cholestasis (Fig. 1). These studies showed that FGF19 is a novel enterokine that is induced via intestinal FXR bile acid activation and functions as an enterohepatic signal in the feedback suppression of bile acid synthesis. Whether altered FGF19 signaling is involved in the differential effects of parenteral lipid emulsions is unclear. However, in the absence of any enteral stimulation from either food or bile acid secretion, it is unlikely that differences in parenteral lipid emulsion FA composition alter gut FGF19 secretion, but this warrants additional examination.
FIGURE 1.
Schematic illustration showing the gut FXR–FGF19 axis and influence of TPN on hepatic CYP7A1 function. CYP7A1, cholesterol 7α-hydroxylase; FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor; RXR, retinoid X receptor; TPN, total parenteral nutrition.
In addition to bile acid homeostasis, FGF19 exerts important regulatory effects on lipid, carbohydrate, and protein metabolism (74, 75). Overexpression or infusion of FGF19 in mice reduces adiposity, serum TGs, and hepatic acetyl-CoA carboxylase while also increasing metabolic rate and glycemic control (76, 77). Conversely, FGFR4-deficient mice exhibit increased white adipose tissue mass, glucose intolerance, insulin resistance, and hyperlipidemia, further confirming an important role of FGF19 in glucose and lipid metabolic regulation (78). Studies in mice also showed that FGF19 functions as a postprandial activator of hepatic protein and glycogen synthesis that is independent of insulin (79).
Parenteral Lipid Emulsions and Vitamin E
A key nutritional component that is also present in most parenteral lipid emulsions that has well-known biologic functions is vitamin E. Vitamin E (tocopherol) is a lipid-soluble antioxidant that protects the integrity of biologic membranes by inhibiting lipid peroxidation (80, 81). Tocopherol occurs as α, β, γ, or δ isoforms, depending on the number and position of methyl groups attached to the chromanol ring. The composition and biologic activity of the different natural vitamin E isoforms varies considerably. The biologic activities of the β, γ, and δ isoforms are 0.5, 0.25, and 0.01, respectively, compared with α-tocopherol (82). Natural α-tocopherol has the highest vitamin E activity given its 3 chiral centers in which methyl groups are in the R configuration and is referred to as RRR-α-tocopherol. The most common form of synthetic vitamin E consists of 8 stereoisomers but has substantially less (12.5%) RRR-α-tocopherol content. The α-tocopherol isomer is found in the highest concentration in human plasma and tissues. Interestingly, plant-derived oils are the most abundant dietary sources of vitamin E and all 4 isoforms are present, but they are mostly enriched with γ-tocopherol (81). Plant germs and seed oils (wheat germ, sunflower seeds, cotton seed, and olive oil) are rich sources of RRR-α-tocopherol (50–100%), whereas γ-tocopherol dominates in soy and corn oil. Thus, the vitamin E present in most commercial soy oil–based emulsions is predominantly the γ-tocopherol isoform (82). However, in several new-generation emulsions, vitamin E is added usually as α-tocopherol isoforms to act as an antioxidant to prevent lipid peroxidation attributable to the high content of LC-PUFA. Lipid peroxidation, the process involving incorporation of an oxygen molecule into the unsaturated FA carbon chain producing lipid peroxides, may occur during parenteral infusion of PUFA-rich lipid emulsions. Lipid peroxides are unstable molecules that, by enzymatic or nonenzymatic decomposition, are converted to volatile malondialdehydes and hydrocarbons that can trigger oxidative stress. Therefore, as a protective measure, the reported α-tocopherol content of some emulsions, including Omegaven, SMOFlipid, and Lipoplus, is up to 4- to 5-fold higher than the γ-tocopherol content of soy-oil emulsions. This combined effect of increased concentration and bioactivity of α-tocopherol in some new-generation emulsions may have substantial biologic effects on hepatic lipid peroxidation and oxidative stress.
Vitamin E and PNALD
In the pathogenesis of fatty liver disease, regardless of whether it occurs in the context of PN (i.e., PNALD) or obesity (i.e., NAFLD), oxidative stress has been proposed as an important cellular process that triggers cell injury and death (31, 39, 40). Oxidative stress results from an imbalance between pro-oxidant and antioxidant chemical species that leads to oxidative damage of cellular macromolecules. The dominant molecules responsible for oxidative stress are products of various oxidative metabolic pathways, collectively referred to as ROS and include singlet oxygen molecules, superoxide anions, hydrogen peroxide, and hydroxyl radicals (40). The increase in ROS triggers additional intracellular damage by causing lipid peroxidation and formation of toxic byproducts, such as trans-4-hydroxy-2-nonenal and malondialdehyde, often used as markers of oxidative stress. Under normal nutritional conditions, mitochondrial β oxidation is the dominant oxidative pathway for disposal of short-, medium-, and long-chain FAs (83). However, when intracellular FAs accumulate, especially very-long-chain FAs (>20 carbon), and overload the mitochondrial β-oxidation pathway, alternate pathways are activated, including peroxisomal β oxidation and microsomal ω oxidation involving enzymes such as acyl-CoA oxidase and cytochrome P450 (CYP4A and CYP4F) (83, 84). Thus, the presence of steatosis during PNALD/NAFLD eventually leads to oxidative stress from overproduction of ROS to dispose of excessive hepatic FA accumulation. Moreover, the accumulation of bile acids associated with cholestasis and PNALD also can induce oxidative stress and thus compound the injury (85).
The cellular evidence linking oxidative stress and injury has prompted a search for antioxidant therapies, including vitamin E, for prevention and treatment of NAFLD and PNALD. Recent large, randomized, controlled trials in adults [PIVENS (Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis)] and pediatric [TONIC (Treatment for NAFLD in Children)] populations have shown that vitamin E treatment results in significant improvement in steatosis, inflammation, ballooning, and resolution of steatohepatitis in adults without diabetes or cirrhosis but provided no sustained benefit in children (86–88). The mechanisms to explain how vitamin E protects against PNALD have been primarily attributed to the cellular antioxidant functions and prevention or reduction of oxidative stress. Additional molecular mechanisms have been postulated whereby vitamin E is involved in the activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR). Both PXR and CAR function as hepatic xenobiotic sensors that, during activation, trigger the expression of a host of drug metabolizing enzyme pathways involving cytochrome P450 and other enzyme systems involved in oxidation, conjugation, sulfation, glucuronidation, and efflux transporters (89–91). The expression of both PXR and CAR and treatment with their selective agonists have been shown to have hepatoprotective actions in various models of cholestatic liver injury, including bile duct ligation and bile acid treatment (92–95). The PXR ligand and antibiotic rifampicin has been shown to suppress CYP7A1 expression. The mechanistic link between vitamin E and PXR was demonstrated by evidence that various stereoisomers of tocopherol and tocotrienol activated PXR–reporter construct and endogenous CYP3A expression in hepatocytes (96, 97). Interestingly, some of the downstream targets of PXR are enzymes systems involved in microsomal ω-oxidation of vitamin E (98). Thus, the hepatoprotective actions of vitamin E treatment in conditions of cholestatic liver injury also may be mediated by activation of PXR and CAR target genes involved in bile acid homeostasis. This could also be a mechanism to explain the protective effects of new-generation lipid emulsions with high levels of added α-tocopherol.
Conclusions
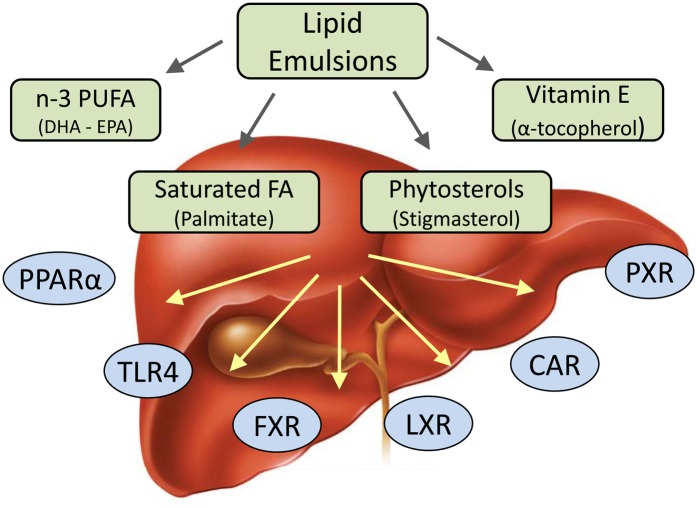
In the past 10 y in Europe and other countries outside the United States, the emergence of new parenteral lipid emulsions containing different source lipids and FA profiles has created an opportunity to optimize the nutritional needs of infants and children requiring PN. In the United States, the soybean oil–based lipid emulsion Intralipid has been the mainstay for PN support for decades, and the new-generation lipid emulsions are only now being considered by the FDA for approval in the United States. Concurrent with these developments has been an expanding scientific understanding about how different lipids and specific FAs affect cellular function and metabolism. The molecular mechanisms whereby cells sense lipids and FAs, namely nuclear receptors, and how these nutrients activate intracellular signaling pathways have rapidly expanded in the past 10 y (Fig. 2) These events have converged and spawned a renewed interest to understand how PN and lipid emulsions, particularly, contribute to or prevent PNALD in infants.
FIGURE 2.
Schematic illustration of lipid emulsion components and receptor signaling pathways reported to influence parenteral nutrition-associated liver disease. CAR, constitutive androstane receptor; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FXR, farnesoid X receptor; LXR, liver X receptor; PPARα, peroxisome proliferator–activated receptor; PXR, pregnane X nuclear receptor; TLR4, toll-like receptor 4.
Clinical studies in infants on prolonged PN suggest that either the practice of lipid emulsion minimization when Intralipid is infused or the complete switch to low-infusion dose of Omegaven can reduce the serum markers of PNALD. The components present in various lipid emulsions that are postulated to contributed to or prevent PNALD include specific FAs, the ratio of n–3 to n–6 LC-PUFA, phytosterols, and vitamin E content. Evidence from clinical studies and those in experimental animals and cell culture suggest that phytosterols are associated with PNALD and that they disrupt the cellular mechanisms of bile acid synthesis and transport by antagonizing hepatocyte FXR. Additional mechanistic studies are required to fully establish how phytosterols affect liver function, the specific cells affected, and disease susceptibility. There also are several reports showing that n–3 LC-PUFA and vitamin E when supplemented in a high-fat diet can reduce measures of NAFLD, such as steatosis, inflammation, and insulin resistance in human and animal studies. However, despite the clinical reports that Omegaven reduces the measures of PNALD induced by Intralipid, there is limited direct information that these effects are mediated by n–3 LC-PUFA or vitamin E specifically. Current literature would suggest that several nuclear receptors involved in lipid sensing are promising candidates, such as PPARα/PPARγ, LXR, and liver receptor homolog 1. In addition, the role of xenobiotic receptors, such as PXR and CAR, may mediate the actions of vitamin E and its metabolites and modulate the function of a host of enzyme systems and transporters involved in liver function.
Additional studies are needed to establish the significance and regulation of the gut FXR–FGF19 signaling axis in the context of PNALD. The lack of intestinal FXR stimulation by enteral bile acids under conditions of TPN may be influenced by the altered gut microbiome as well, because bacterial play an influential role in bile acid metabolism. Short-bowel syndrome is a clinical condition frequently associated with PNALD, and substantial loss of intestinal tissue may limit the capacity of the gut FXR–FGF19 signaling axis and contribute to cholestatic liver injury. The capacity to activate the gut FXR–FGF19 signaling axis is also influenced by the chemical form of bile acids, and groups are actively developing analogs that selectively activate FXR, e.g., obeticholic acid, and that may be more effective in treating cholestatic liver diseases than those used previously, such as ursodeoxycholic acid, that are weak FXR agonists (99).
Some of the hurdles that have delayed research in the United States on parenteral lipid emulsions in experimental animal models in vivo stem from the availability of commercial lipid emulsions and technical challenges of modifying the physiochemical composition of FAs and particles while maintaining compatibility for intravenous infusion. Furthermore, the application of PN approaches in mice has been technically challenging, but recent reports suggest that these issues are being resolved so that the power of mouse genetic models can be used to explore molecular mechanisms (57, 100). New developments to established pig models will also facilitate lipid emulsion studies in a variety of clinically relevant conditions that affect pediatric liver disease, such as prematurity, short-bowel syndrome, and necrotizing enterocolitis (101–103).
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BSEP, bile salt export pump; CAR, constitutive androstane receptor; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7α-hydroxylase; FGF15/19, fibroblast growth factor 15/19; FGFR4, FGF receptor 4; FXR, farnesoid X receptor; LC-PUFA, long-chain PUFA; LXR, liver X receptor; NAFLD, nonalcoholic fatty liver disease; PN, parenteral nutrition; PNALD, parenteral nutrition-associated liver disease; PPAR, peroxisome proliferator–activated receptor; PXR, pregnane X nuclear receptor; ROS, reactive oxygen species; TPN, total parenteral nutrition; TLR4, toll-like receptor 4.
Literature Cited
- 1.Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27:284–90 [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002;29:225–44 [DOI] [PubMed] [Google Scholar]
- 4.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:277–87 [DOI] [PubMed] [Google Scholar]
- 5.Zambrano E, El Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Mugica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol. 2004;7:425–32 [DOI] [PubMed] [Google Scholar]
- 6.Driscoll DF, Bistrian BR, Demmelmair H, Koletzko B. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clin Nutr. 2008;27:497–503 [DOI] [PubMed] [Google Scholar]
- 7.Cober MP, Killu G, Brattain A, Welch KB, Kunisaki SM, Teitelbaum DH. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160:421–7 [DOI] [PubMed] [Google Scholar]
- 8.Diamond IR, de Silva NT, Tomlinson GA, Pencharz PB, Feldman BM, Moore AM, Ling SC, Wales PW. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: a multiple-variable analysis. JPEN J Parenter Enteral Nutr. 2011;35:596–602 [DOI] [PubMed] [Google Scholar]
- 9.Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, Puder M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201 [DOI] [PubMed] [Google Scholar]
- 10.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15 [DOI] [PubMed] [Google Scholar]
- 11.de Meijer VE, Gura KM, Meisel JA, Le HD, Puder M. Parenteral fish oil as monotherapy for patients with parenteral nutrition-associated liver disease. Pediatr Surg Int. 2009;25:123–4 [DOI] [PubMed] [Google Scholar]
- 12.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86 [DOI] [PubMed] [Google Scholar]
- 14.Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–84 [DOI] [PubMed] [Google Scholar]
- 15.Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83:1520S–5S [DOI] [PubMed] [Google Scholar]
- 16.Harris WS, Colombo J, Toner CD, Lefevre M, Mozaffarian D, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–19S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159:743–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, Omenetti A, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–96 [DOI] [PubMed] [Google Scholar]
- 20.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jump DB, Thelen A, Ren B, Mater M. Multiple mechanisms for polyunsaturated fatty acid regulation of hepatic gene transcription. Prostaglandins Leukot Essent Fatty Acids. 1999;60:345–9 [DOI] [PubMed] [Google Scholar]
- 22.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S [DOI] [PubMed] [Google Scholar]
- 23.Yaqoob P, Calder PC. Fatty acids and immune function: new insights into mechanisms. Br J Nutr. 2007;98(Suppl 1):S41–5 [DOI] [PubMed] [Google Scholar]
- 24.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403 [DOI] [PubMed] [Google Scholar]
- 26.Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–32 [DOI] [PubMed] [Google Scholar]
- 27.Duval C, Muller M, Kersten S. PPARalpha and dyslipidemia. Biochim Biophys Acta. 2007;1771:961–71 [DOI] [PubMed] [Google Scholar]
- 28.Gervois P, Fruchart JC, Staels B. Inflammation, dyslipidaemia, diabetes and PPars: pharmacological interest of dual PPARalpha and PPARgamma agonists. Int J Clin Pract Suppl. 2004;(143):22–9 [DOI] [PubMed] [Google Scholar]
- 29.Hall RI, Grant JP, Ross LH, Coleman RA, Bozovic MG, Quarfordt SH. Pathogenesis of hepatic steatosis in the parenterally fed rat. J Clin Invest. 1984;74:1658–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25 [DOI] [PubMed] [Google Scholar]
- 31.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88 [DOI] [PubMed] [Google Scholar]
- 32.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–41 [DOI] [PubMed] [Google Scholar]
- 33.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93 [DOI] [PubMed] [Google Scholar]
- 34.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13–24 [DOI] [PubMed] [Google Scholar]
- 35.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazanave SC, Gores GJ. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5:71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94 [DOI] [PubMed] [Google Scholar]
- 38.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–34 [DOI] [PubMed] [Google Scholar]
- 40.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–101 [DOI] [PubMed] [Google Scholar]
- 42.Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–30 [DOI] [PubMed] [Google Scholar]
- 43.Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr. 2010;140:2193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2012;302:G218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91 [DOI] [PubMed] [Google Scholar]
- 46.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geier A, Fickert P, Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:574–85 [DOI] [PubMed] [Google Scholar]
- 48.Mulder J, Karpen SJ, Tietge UJ, Kuipers F. Nuclear receptors: mediators and modifiers of inflammation-induced cholestasis. Front Biosci (Landmark Ed). 2009;14:2599–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturm E, Wagner M, Trauner M. Nuclear receptor ligands in therapy of cholestatic liver disease. Front Biosci (Landmark Ed). 2009;14:4299–325 [DOI] [PubMed] [Google Scholar]
- 50.Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis. 2010;30:186–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565–80 [DOI] [PubMed] [Google Scholar]
- 52.Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–95 [DOI] [PubMed] [Google Scholar]
- 53.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–70 [DOI] [PubMed] [Google Scholar]
- 55.Kubota A, Okada A, Imura K, Kawahara H, Nezu R, Kamata S, Takagi Y. The effect of metronidazole on TPN-associated liver dysfunction in neonates. J Pediatr Surg. 1990;25:618–21 [DOI] [PubMed] [Google Scholar]
- 56.Freund HR, Muggia-Sullam M, LaFrance R, Enrione EB, Popp MB, Bjornson HS. A possible beneficial effect of metronidazole in reducing TPN-associated liver function derangements. J Surg Res. 1985;38:356–63 [DOI] [PubMed] [Google Scholar]
- 57.El Kasmi KC, Anderson AL, Devereaux MW, Fillon SA, Harris JK, Lovell MA, Finegold MJ, Sokol RJ. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology. 2012;55:1518–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86 [DOI] [PubMed] [Google Scholar]
- 59.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–64 [DOI] [PubMed] [Google Scholar]
- 61.Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 1993;105:1806–13 [DOI] [PubMed] [Google Scholar]
- 62.Ellegård L, Sunesson A, Bosaeus I. High serum phytosterol levels in short bowel patients on parenteral nutrition support. Clin Nutr. 2005;24:415–20 [DOI] [PubMed] [Google Scholar]
- 63.Kurvinen A, Nissinen MJ, Gylling H, Miettinen TA, Lampela H, Koivusalo AI, Rintala RJ, Pakarinen MP. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J Pediatr Gastroenterol Nutr. 2011;53:440–6 [DOI] [PubMed] [Google Scholar]
- 64.Kurvinen A, Nissinen MJ, Andersson S, Korhonen P, Ruuska T, Taimisto M, Kalliomaki M, Lehtonen L, Sankilampi U, Arikoski P, et al. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. J Pediatr Gastroenterol Nutr. 2012;54:803–11 [DOI] [PubMed] [Google Scholar]
- 65.Bindl L, Lutjohann D, Buderus S, Lentze MJ, Bergmann K. High plasma levels of phytosterols in patients on parenteral nutrition: a marker of liver dysfunction. J Pediatr Gastroenterol Nutr. 2000;31:313–6 [DOI] [PubMed] [Google Scholar]
- 66.Llop JM, Virgili N, Moreno-Villares JM, Garcia-Peris P, Serrano T, Forga M, Solanich J, Pita AM. Phytosterolemia in parenteral nutrition patients: implications for liver disease development. Nutrition. 2008;24:1145–52 [DOI] [PubMed] [Google Scholar]
- 67.Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–6 [DOI] [PubMed] [Google Scholar]
- 68.El Kasmi KC, Anderson AL, Devereaux MW, Vue PM, Zhang W, Setchell KD, Karpen SJ, Sokol RJ. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. 2013. ;5:206ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25 [DOI] [PubMed] [Google Scholar]
- 71.Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di TG, Palasciano G, Moustafa T, Halilbasic E, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–65 [DOI] [PubMed] [Google Scholar]
- 72.Dawson PA, Hubbert ML, Rao A. Getting the mOST from OST: role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism. Biochim Biophys Acta. 2010;1801:994–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundåsen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–6 [DOI] [PubMed] [Google Scholar]
- 74.Kuro-o M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol Metab. 2008;19:239–45 [DOI] [PubMed] [Google Scholar]
- 75.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93 [DOI] [PubMed] [Google Scholar]
- 76.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–603 [DOI] [PubMed] [Google Scholar]
- 77.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–7 [DOI] [PubMed] [Google Scholar]
- 78.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes. 2007;56:2501–10 [DOI] [PubMed] [Google Scholar]
- 79.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–62 [DOI] [PubMed] [Google Scholar]
- 81.Biesalski HK. Vitamin E requirements in parenteral nutrition. Gastroenterology. 2009;137:S92–104 [DOI] [PubMed] [Google Scholar]
- 82.Wanten GJ, Roos D, Naber AH. Effects of structurally different lipid emulsions on human neutrophil migration. Clin Nutr. 2000;19:327–31 [DOI] [PubMed] [Google Scholar]
- 83.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230 [DOI] [PubMed] [Google Scholar]
- 84.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263–75 [DOI] [PubMed] [Google Scholar]
- 85.Sokol RJ, Devereaux M, Dahl R, Gumpricht E. “Let there be bile”–understanding hepatic injury in cholestasis. J Pediatr Gastroenterol Nutr. 2006;43:(Suppl 1):S4–9 [DOI] [PubMed] [Google Scholar]
- 86.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:641–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46 [DOI] [PubMed] [Google Scholar]
- 90.Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62:1238–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr. 2003;133:2444S–7S [DOI] [PubMed] [Google Scholar]
- 92.Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71 [DOI] [PubMed] [Google Scholar]
- 94.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teng S, Piquette-Miller M. Regulation of transporters by nuclear hormone receptors: implications during inflammation. Mol Pharm. 2008;5:67–76 [DOI] [PubMed] [Google Scholar]
- 96.Traber MG. Vitamin E, nuclear receptors and xenobiotic metabolism. Arch Biochem Biophys. 2004;423:6–11 [DOI] [PubMed] [Google Scholar]
- 97.Landes N, Pfluger P, Kluth D, Birringer M, Ruhl R, Bol GF, Glatt H, Brigelius-Flohe R. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65:269–73 [DOI] [PubMed] [Google Scholar]
- 98.Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann N Y Acad Sci. 2004;1031:13–21 [DOI] [PubMed] [Google Scholar]
- 99.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, et al. Efficacy and safety of the farnesoid x receptor agonist obeticholic Acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82 [DOI] [PubMed] [Google Scholar]
- 100.Tazuke Y, Drongowski RA, Btaiche I, Coran AG, Teitelbaum DH. Effects of lipid administration on liver apoptotic signals in a mouse model of total parenteral nutrition (TPN). Pediatr Surg Int. 2004;20:224–8 [DOI] [PubMed] [Google Scholar]
- 101.Turner JM, Wales PW, Nation PN, Wizzard P, Pendlebury C, Sergi C, Ball RO, Pencharz PB. Novel neonatal piglet models of surgical short bowel syndrome with intestinal failure. J Pediatr Gastroenterol Nutr. 2011;52:9–16 [DOI] [PubMed] [Google Scholar]
- 102.Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130:1776–92 [DOI] [PubMed] [Google Scholar]
- 103.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28:210–22 [DOI] [PubMed] [Google Scholar]