Figure 2.
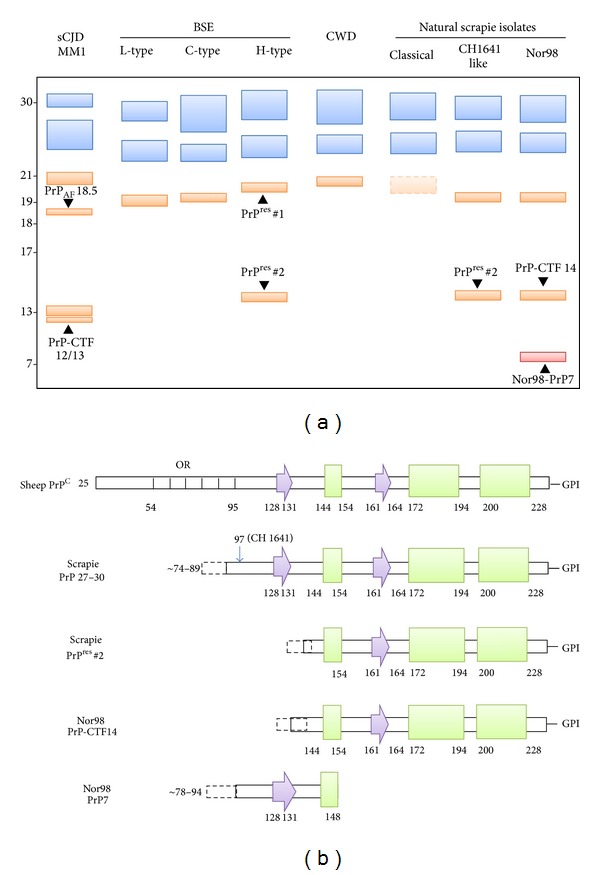
(a) Schematic representation of the spectrum of PrPres fragments observed in animal prion diseases and their electrophoretic profile. The unglycosylated forms of all PrPres fragments with the glycosylation sites in their sequence are indicated in orange, while the fragments lacking these sites are shown in red. Among the glycosylated peptides, only the mono- and the diglycosylated forms of PrPres 27–30 (18–21 kDa range) fragments are shown (in blue). To facilitate the comparison with human forms, the profile of MM1 sCJD associated PrPres is shown; note that the unglycosylated band of sCJDMM1 PrPres has the same electrophoretic mobility of that of CWD as reported by Xie et al. [14]. (b) Diagrams of the secondary structural elements of sheep PrPC and of the PK-resistant PrP fragments observed in classical and atypical Nor98 scrapie. Arrows are representative of β-strands and rectangles of α-helices and OR indicates the octapeptide repeats region. The secondary structure numbering has been derived from pdb (Protein Data Bank) id 1XYU (sheep PrP).
