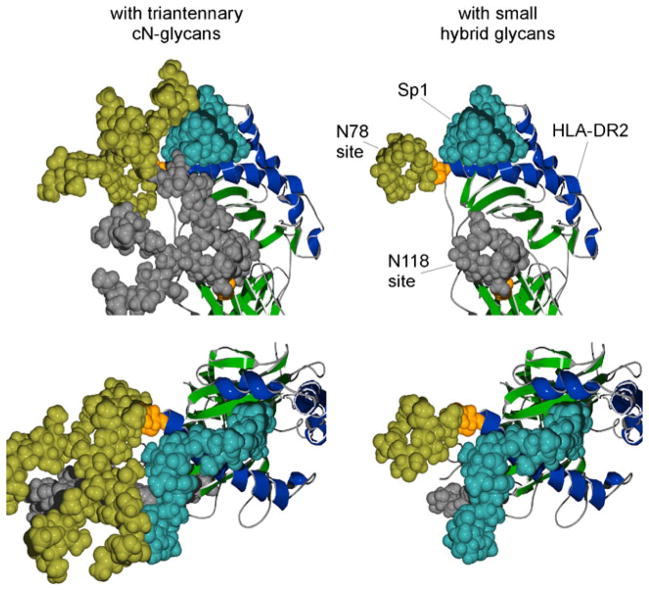
Fig. 6.
Structural model of Sp1 glycoantigen bound to human HLA-DR2 in the presence or absence of complex N-glycans. Images were created using previously defined crystal structures of HLA-DR2 [122] with modeled N-glycan structures (GLYCAM web tool [123]). The upper and lower images are showing different views of Sp1 (coordinates provided by Julia Wang [89]) associated with HLA-DR2 in the presence of attached triantennary complex N-glycans (left) or hybrid N-glycans (right). Alpha helices of the antigen binding groove are shaded blue and the rest of the molecule in green. The alpha chain N-glycan acceptor sites N78 and N118 are shown in orange as space-filled residues with the attached N-glycan structures shown in yellow and gray, respectively. The predicted association of a glycoantigen (Sp1 from S. pneumoniae; blue) is shown