Abstract
Objective
Eosinophilic oesophagitis (EoE) is a chronic inflammatory condition of the oesophagus with limited treatment options. No previous transgenic model has specifically targeted the oesophageal mucosa to induce oesophageal eosinophilia.
Design
We developed a mouse model that closely resembles EoE by utilising oxazolone haptenation in mice with transgenic overexpression of an eosinophil poietic and survival factor (interleukin (IL)-5) in resident squamous oesophageal epithelia.
Results
Overexpression of IL-5 in the healthy oesophagus was achieved in transgenic mice (L2-IL5) using the squamous epithelial promoter Epstein–Barr virus ED-L2. Oxazolone-challenged L2-IL5 mice developed dose-dependent pan-oesophageal eosinophilia, including eosinophil microabscess formation and degranulation as well as basal cell hyperplasia. Moreover, oesophagi expressed increased IL-13 and the eosinophil agonist chemokine eotaxin-1. Treatment of these mice with corticosteroids significantly reduced eosinophilia and epithelial inflammation.
Conclusions
L2-IL5 mice provide a novel experimental model that can potentially be used in preclinical testing of EoE-related therapeutics and mechanistic studies identifying pathogenetic features associated with mucosal eosinophilia.
INTRODUCTION
Eosinophilic oesophagitis (EoE) is a chronic allergic gastrointestinal disease manifest by dysphagia and food impaction in adults as well as a collection of symptoms previously associated with gastro-oesophageal reflux disease in children.1,2 Diagnostic criteria focus on clinical and histopathological assessments that centre on the presence of eosinophils in the oesophageal epithelia.1
EoE occurs as a result of a hypersensitivity response in the oesophageal mucosa. As such, currently available mouse models of EoE have been derived by the direct allergen challenge of oesophageal mucosae following sensitisation in systemic transgenic mice.3–7 This approach derives from observations that elimination of food allergens benefits clinicopathological aspects of the disease,8,9 and there is increased local expression of eosinophil agonists, including interleukin (IL)-5 and eotaxin chemokines.5,10–14 These mouse models amplify the local response with systemic overexpression of the eosinophil agonist cytokine IL-5 (CD2-IL5)5 or ectopic constitutive expression in non-oesophageal organs such as the lung (CC10-IL-13)15 and small intestine (FABPi-IL-5, FABPi-Eotaxin).4,16
In the present study, we took an alternative approach and specifically targeted IL-5 expression to the oesophageal squamous epithelia using the Epstein–Barr virus ED-L2 (EBV-ED-L2) gene promoter.17 These data showed that constitutive squamous oesophageal epithelial-specific overexpression of IL-5 (L2-IL5 transgenic mice) resulted in a baseline oesophageal eosinophilia. On sensitisation and subsequent topical oesophageal challenge of L2-IL5 mice with oxazolone, a molecule known to induce T-helper cell (Th) type 2 responses in other organs, mice developed key histopathological features consistent with human EoE. These features include epithelial hyperplasia and eosinophilic microabscesses that erupt through the epithelium into the oesophageal lumen. This model will provide investigators with a novel system to determine mechanistic pathways and test preclinical therapeutics focusing on EoE.
MATERIALS AND METHODS
Generation of L2-IL5 transgenic mice
The expression of a 5.5 kb IL-5 complementary DNA/genomic fusion gene18 was driven using a promoter derived from the EBV-ED-L2 gene17,19 (figure 1). Age-matched wild-type C57BL/6J littermate mice were used as controls for all experiments. Mouse studies were approved by the University of Colorado Denver Institutional Animal Care and Use Committee.
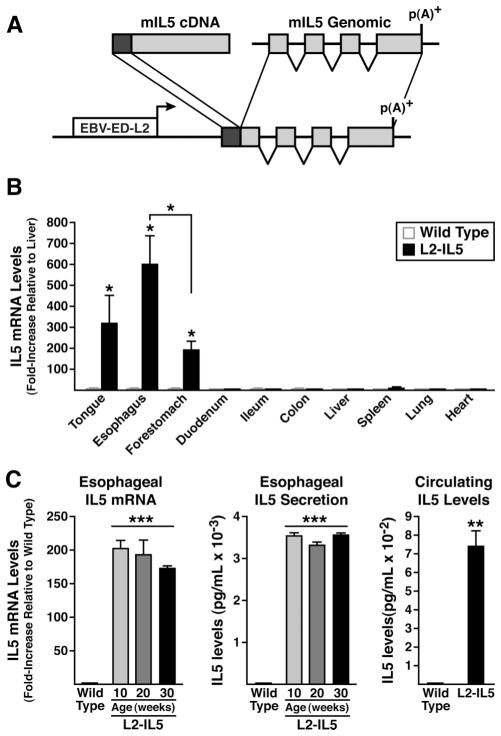
Figure 1.
IL-5 transgenic mice utilising the Epstein–Barr virus ED-L2 (EBV-ED-L2) gene promoter generate animals that ectopically express high levels of IL-5 from squamous epithelium of the oesophagus. (A) An EBV-ED-L2 promoter fragment was cloned upstream of mouse IL-5 cDNA:genomic fusion gene that includes the complete IL-5 ORF and all of the IL-5 genomic exons/introns without any endogenous upstream cis-regulatory sequences. (B) IL-5 transgene expression (ie, mRNA) assessed in squamous epithelial-derived tissues (reverse transcription (RT)–PCR) demonstrates a unique pattern of induced ectopic expression with maximum in the oesophagus. IL-5 mRNA levels in a given tissue were initially normalised to 18S within each sample and expression levels among tissues examined are reported as fold increase relative to IL-5 expression in liver (ie, a tissue devoid of squamous-epithelium). (C) IL-5 mRNA (oesophageal) and protein (oesophageal and circulating) levels in L2-IL5 mice are characteristic of a pattern of localised IL-5 overexpression with displacement into systemic circulation. Oesophagi from wild-type and L2-IL5 mice (10–30 weeks of age) were processed for whole tissue RNA and assessed for IL-5 expression by RT-PCR. IL-5 levels were normalised to 18S within each sample and then reported as fold-increase relative to wild-type levels at 10 weeks of age. IL-5 secretion from the oesophagus was assessed by ELISA from supernatants of whole oesophageal explant cultures and circulating IL-5 levels were determined from serum samples derived from blood recovered from 10-week-old wild-type and L2-IL5 mice. These data are expressed as means±SEM of five to six individual mice per group. *p≤0.05, **p≤0.01, ***p≤0.001.
Induction of experimental oesophageal eosinophilia in L2-IL5 mice by sensitisation and topical challenge with oxazolone
A mouse model of oesophageal inflammation was established using a 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA; Sigma, St Louis, Missouri, USA) contact hypersensitivity protocol (figure 2). See supplementary materials (available online only).20
Figure 2.
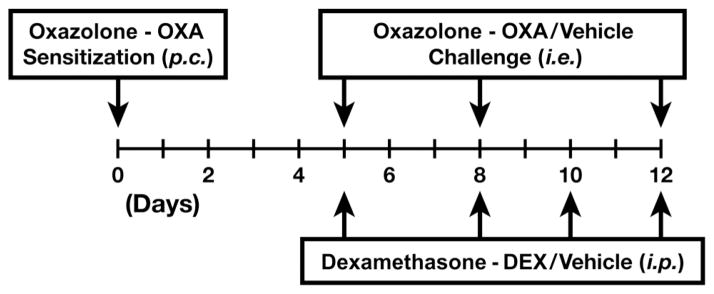
Schematic timeline representing the 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) sensitisation/topical challenge model of delayed contact hypersensitivity. On day 0 of the OXA hypersensitivity protocol, a 2 cm×2 cm area of abdominal skin of anaesthetised experimental animals was shaved and OXA was applied to the skin surface (150 μl of a 3% (w/v) solution of OXA in 4:1 acetone-olive oil vehicle) to initiate the sensitisation phase of the protocol.20 Sensitised mice received topical OXA challenges of the oesophagus by gavage (p.c.) on protocol days 5, 8 and 12 with a 1% (w/v) solution of OXA in 30% ethanol/olive oil vehicle into the proximal oesophagus. Vehicle control animals (either wild-type or L2-IL5 mice) were sensitised as noted above and challenged with 4:1 acetone-olive oil vehicle alone. All mice were assessed 24 h following the last OXA challenge (protocol day 13). Additional groups of mice were concurrently treated with the corticosteroid dexamethasone (DEX) by intraperitoneal (i.p.) injection of mice (10 mg/kg of body weight) during the topical OXA challenge phase (protocol days 5, 8, 10 and 12); control animals receiving intraperitoneal injections of saline vehicle alone.
Dexamethasone treatment
Dexamethasone treatments were performed as described21 (figure 2). See supplementary materials (available online only).
Tissue collection and histological analysis
Tissues were fixed with 10% neutral buffered formalin and processed in preparation for being paraffin embedded.21 Serial sections from these paraffin tissue blocks were cut (5 μm) and were either stained with H&E or subjected to histological assessments (see table 1) or immunohistochemistry using either an anti-mouse eosinophil major basic protein (MBP)-specific rat monoclonal antibody,22 the Ki67 monoclonal antibody to assess cell proliferation.23 Quantification of either MBP-1 (ie, eosinophils) or Ki67 immunopositive cells are presented as numerical averages of nine non-overlapping high-power fields (0.26 mm2) per oesophagus (three distal, three mid and three proximal). Slides were also subjected to immunofluorescence analysis. See supplementary materials (available online only).
Table 1.
Epithelial Histology Scoring Tool
| Epithelial histology scoring tool | Score |
|---|---|
| Eosinophilic clusters/abscesses | 0=Absent |
| 1=Present | |
| Lymphoplasmacytic infiltrates | 0=Absent |
| 1=Present | |
| Hyperkeratosis | 0=Absent |
| 1=Present | |
| Dilated intercellular spaces | 0=Absent |
| 1=Present | |
| Basal zone hyperplasia | 0=≤25% Epithelial thickness |
| 1=25–50% Epithelial thickness | |
| 2=50–75% Epithelial thickness | |
| 3=>75% Epithelial shickness |
Cell isolation, quantification and flow cytometric and fluorescence-activated cell-sorting analysis
Leucocytes from tissue sources including spleen, bone marrow, peripheral blood and oesophagus were isolated as previously described.21,23,24 See supplementary materials (available online only).
Single-cell suspensions were stained on ice with cell type-specific antibodies as previously described.21 See supplementary materials (available online only).
RNA isolation and real-time reverse transcription PCR
Whole oesophageal tissue total RNA was prepared using RNeasy mini kits (Qiagen, Valencia, California, USA), cDNA synthesis (Applied BioSystems) and real-time reverse transcription (RT)–PCR with Taqman gene expression assays Taqman probes (Applied Biosystems) as previously described.21
Cytokine assessments of oesophageal tissue and blood serum
Oesophagi and blood serum were assessed for cytokine production as previously described.21,23
Statistical analysis
Statistical analyses of data outcomes were performed using a two-tailed Student’s t test. Data are expressed as means±SEM. A p value of 0.05 or less was considered statistically significant, although in some cases higher levels of significance are noted and described in the figure legends when applicable.
RESULTS
Transgenic mice constitutively express IL-5 from oesophageal squamous epithelial cells
A mouse model replicating inflammatory changes linked with EoE was developed by generating transgenic mice (L2-IL5) that constitutively express the eosinophil agonist cytokine IL-5 in resident oesophageal stratified squamous epithelial cells (figure 1A). The tissue/cell specificity of ectopic IL-5 expression in these mice was achieved using an Epstein–Barr virus (ED-L2) promoter fragment that was previously shown to limit heterologous gene expression to tissues/organs that contain squamous epithelium.17 RT–PCR assessments of IL-5 gene expression in L2-IL5 mice thus demonstrated that transgene expression is limited to the tongue, oesophagus and forestomach, with the highest level of IL-5 expression found in the oesophagus (figure 1B). These RT–PCR assessments also showed that oesophageal IL-5 expression in L2-IL5 mice did not vary as a function of postpartum age (wild-type control animals displayed little to no IL-5 expression at any time point examined), remaining at constitutively high levels from 10 weeks to 30 weeks of age (figure 1C).
Production of IL-5 protein from the oesophagus was confirmed by ELISA assessment of culture supernatants derived from oesophageal explants. Consistent with observed levels of messenger RNA/gene expression, IL-5 secretion by oesophageal explants also did not vary as a function of postpartum age (figure 1C). Similar to a pulmonary model of IL-5 overexpression,25 serum IL-5 in the L2-IL5 mice was elevated (figure 1C).
Systemic and oesophageal IL-5 overexpression induce eosinophilia in haematopoetic, peripheral and oesophageal compartments
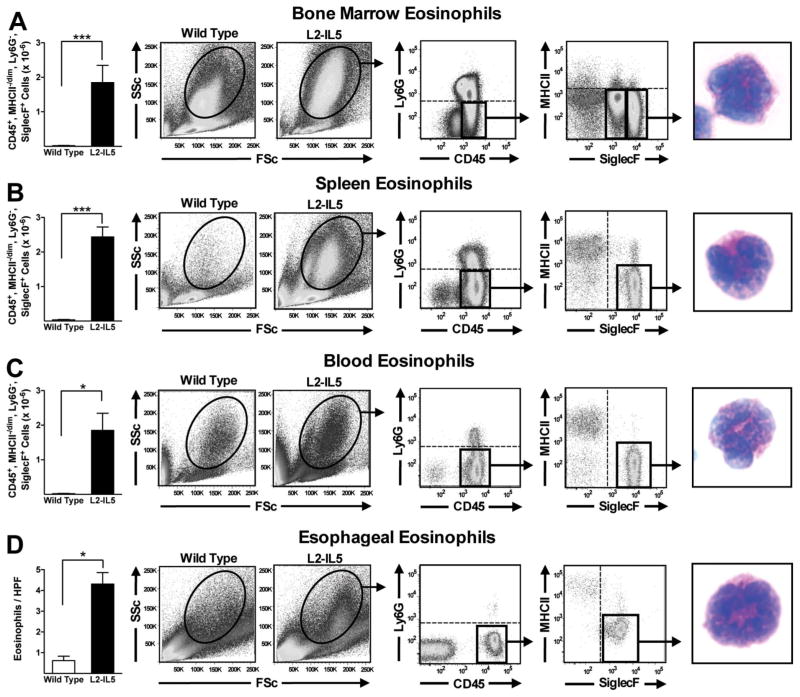
Elevated IL-5 levels in the peripheral circulation of 10-week-old L2-IL5 mice were associated with significant increases (ie, relative to wild-type control animals) in eosinophil levels in haematopoietic compartments including the bone marrow (figure 3A) and spleen (figure 3B). In both cases cytospin assessment of DiffQuick (commercial Romanowsky) stained slides revealed cells with the characteristic appearance of eosinophil-committed progenitor cells. Additional evidence of extramedullary haematopoiesis in L2-IL5 mice also included splenomegaly and splenic eosinophil clusters by routine H&E histology (see supplementary figure S1, available online only). Assessments of peripheral blood (figure 3C) showed that unlike the nominal circulating levels of white blood cells and eosinophils found in wild-type control animals (1.37×10−6 cells and 0.01×10−6 eosinophils, respectively), L2-IL5 mice displayed significant increases in both white blood cells and peripheral eosinophil numbers (5.9×10−6 cells and 1.8×10−6 eosinophils, respectively); DiffQuick (commercial Romanowsky) stains of cytospins showed that these cells displayed morphologies characteristic of mature peripheral eosinophils.
Figure 3.
Constitutive expression of IL-5 from the squamous epithelium of L2-IL5 elicits the expansion of the eosinophilopoietic compartments of bone marrow and spleen, an increase in circulating eosinophil numbers and an oesophageal eosinophilia. Flow cytometric analysis in combination with fluorescence-activated cell sorting (FACS) was performed to identify and quantify the induced eosinophilia occurring in L2-IL5 mice. Regardless of the tissue/compartment examined eosinophils were identified through sequential gates initially focusing on forward versus side scatter before employing specific gates based on the presence/absence of unique cell surface markers (ie, CD45+, Ly6G−, MHCII−/dim, SiglecF+). Therefore, eosinophils in (A) bone marrow, (B) spleen, (C) peripheral blood and (D) oesophagus were identified and quantified in 10-week-old L2-IL5 mice relative to wild-type controls. These cells were subsequently subjected to FACS and eventually cell differential analysis of DiffQuick (commercial Romanowsky) stained cytospin preparations of the sorted cell populations, identifying eosinophils in each of these compartments/tissues. These data are expressed as means±SEM of five to six individual mice per group. *p≤0.05 and ***p≤0.001. This figure is only reproduced in colour in the online version.
Detailed assessments of the oesophagi of L2-IL5 mice showed that the localised squamous epithelial cell expression of IL-5 resulted in prominent oesophageal tissue eosinophilia (figure 3D). In particular, MBP-1 demonstrated a significant increase in tissue infiltrating eosinophils in this compartment relative to wild-type control animals (4.3±0.6 eosinophils/400× high powered field (hpf) (in all microscopic examinations, hpf=0.26mm2) versus 0.6 ±0.2 eosinophils/400× hpf. These assessments of stained tissue sections were confirmed by fluorescence-activated cell-sorting analyses of disassociated cells recovered from oesophagi (figure 3D). These data also showed that the oesophagus of an L2-IL5 mouse displays a significant increase (ie, relative to wild type) in resident eosinophils (ie, CD45+, Ly6G−, MHC II−/dim, Siglec F+ cells; 5.7×10−3 vs 0.16×10−3) whose appearance on DiffQuick (commercial Romanowsky) staining is that of mature eosinophilic metamyelocytes. In addition, a small and moderate increase in the presence of neutrophils (CD45+, CD11b+, Ly6G+; 0.14×10−3 vs 0.017×10−3), dendritic cells (CD45+, CD11c+, MHCII+; 0.38×10−3 vs 0.23×10−3), macrophages (CD45+, CD11b+, MHCII+; 2.12×10−3 vs 0.82×10−3), CD4+ (2.4×10−3 vs 0.63×10−3) and CD8+ (0.59×10−3 vs 0.13×10−3) T lymphocytes were identified by flow cytometry in the oesophagi of L2-IL5 mice compared to wild-type controls. Mast cells were assessed by alcian blue pH2.5 immunohistochemistry and were not considered a significant component in the oesophagi of L2-IL5 mice.
OXA sensitisation and oesophageal challenge of L2-IL5 mice resulted in eosinophil-dominated oesophageal inflammation consistent with EoE
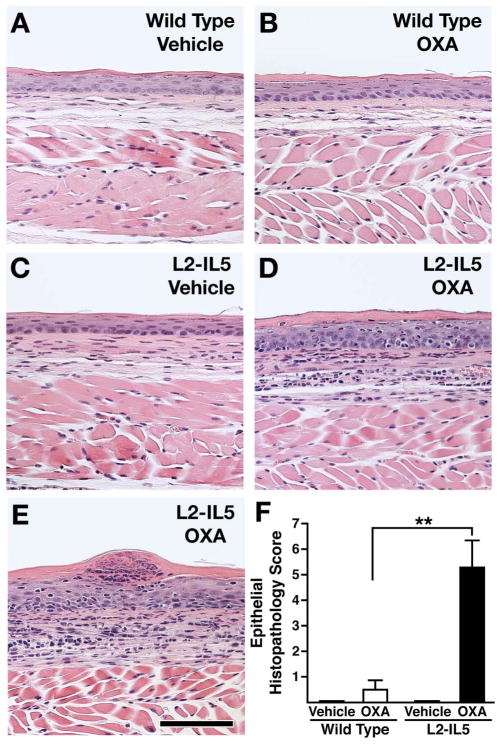
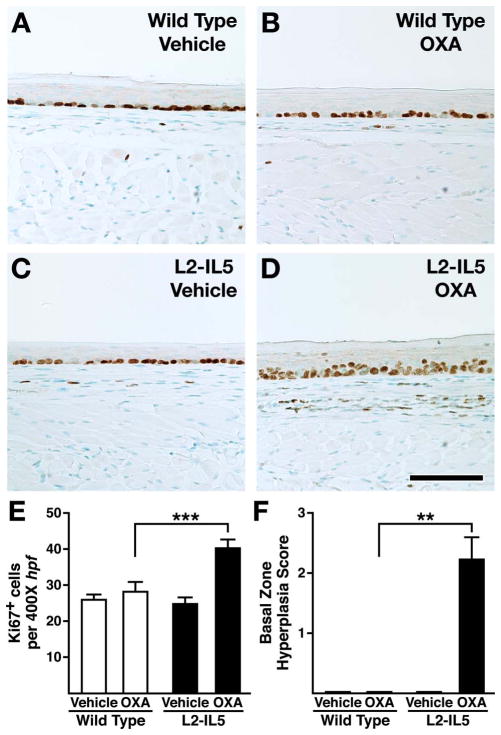
Previous studies utilising OXA-induced delayed type hypersensitivity reactions in the large intestine and skin have successfully induced a robust Th2 and eosinophil-associated inflammatory pathology.26,27 We hypothesised that a localised delayed hypersensitivity reaction induced in mice predisposed to eosinophilic oesophageal inflammation (ie, L2-IL5 animals) would provide the necessary immune-mediated response to replicate the histopathologies characteristically found in EoE patients. L2-IL5 mice were sensitised with OXA and then subjected to a topical OXA challenge of the oesophagus to induce a localised oesophageal delayed hypersensitivity response (figure 2). OXA sensitisation and topical oesophageal challenge of wild-type mice induced a nominal increase in oesophageal epithelial histopathology relative to vehicle control animals that did not achieve statistical significance (figure 4A,B). In contrast, OXA sensitisation and topical oesophageal challenge of L2-IL5 animals resulted in histopathological changes of the oesophageal epithelium that were significantly higher than either those displayed by L2-IL5 animals treated with vehicle alone or OXA-sensitised/challenged wild-type mice (figure 4C–E). Representative photomicrographs of H&E oesophageal sections demonstrated the induced expansion of the oesophageal epithelium and inflammatory cell infiltrate occurring in OXA-treated L2-IL5 mice (figure 4D,E) relative to either vehicle-treated L2-IL5 animals (figure 4C) or vehicle versus OXA-treated wild-type mice (figure 4A vs B, respectively), findings confirmed in histopathology scores (figure 4F).
Figure 4.
4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) sensitisation and topical oesophageal challenge of L2-IL5 mice results in histopathological changes consistent with those observed in humans with eosinophilic oesophagitis (EoE). H&E stained sections of oesophagus were evaluated and representative photomicrographs are presented from wild-type animals treated either with (A) vehicle or (B) OXA and L2-IL5 mice treated either with (C) cehicle or (D, E) OXA. (F) The peak level of inflammation (epithelial histopathology score) in each group of mice was determined using an algorithm described in the supplementary materials (available online only) and expressed as group mean data±SEM. Scale bar represents 100 μM. These data are representative of three separate experiments with five to nine individual mice per group. **p≤0.01. This figure is only reproduced in colour in the online version.
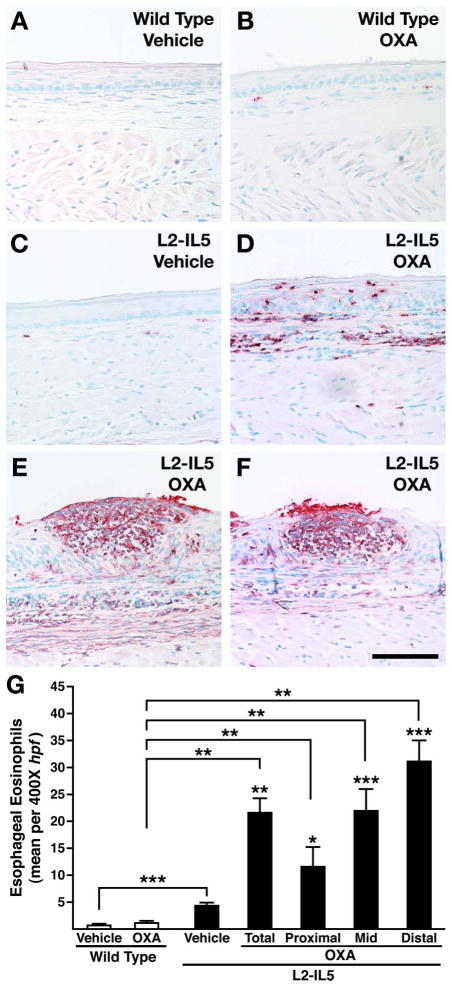
The extent of the induced oesophageal eosinophilia and evidence of eosinophil degranulation was determined by immunohistochemical detection of the eosinophil secondary granule MBP-1.21 These immunohistochemical assessments revealed that OXA-treated wild-type mice displayed mild tissue eosinophilia relative to the virtual absence of eosinophils in vehicle-alone-treated wild-type animals (figure 5A,B). In addition, these data showed that while the eosinophilia occurring in L2-IL5 vehicle-alone-treated mice (figure 5C) was statistically larger than those observed in wild-type animals, this response was dwarfed by the induced eosinophilia and associated degranulation occurring in OXA-treated L2-IL5 mice (figure 5D–F). The identity of MBP-1+ cells in OXA-treated L2-IL5 mice as exclusively eosinophil lineage in origin was confirmed histologically by double immunofluorescence staining of oesophageal tissue sections for Siglec F and MBP-1 (see supplementary figure S2, available online only). The extent of induced eosinophilia in wild-type versus L2-IL5 OXA-treated mice was quantified (figure 5G) by detailed examination of tissue sections (n=5–10/cohort) as the mean number of MBP-1+ cells/400× hpf. These data showed that wild-type (vehicle control or OXA-treated) and vehicle control L2-IL5 mice displayed only a nominal oesophageal eosinophilia (averaged over the total oesophageal length) relative to the induced eosinophilia occurring in OXA-treated L2-IL5 mice. Quantification of induced tissue eosinophilia occurring in OXA-treated L2-IL5 mice demonstrated a longitudinal gradient that occurred with a statistically higher density of eosinophils in distal oesophagi (figure 5G). No significant change in other leucocyte populations was observed following OXA challenge of L2-IL5 mice.
Figure 5.
4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) sensitisation and topical oesophageal challenge of L2-IL5 mice results in eosinophilic histopathological changes consistent with those observed in humans with eosinophilic oesophagitis (EoE). Oesophageal eosinophil infiltration and localised degranulation were determined by immunohistochemistry using a rat monoclonal anti-mouse major basic protein (MBP) antibody. Stained oesophageal sections were evaluated and representative photomicrographs are presented from wild-type animals treated either with (A) vehicle or (B) OXA and L2-IL5 mice treated either with (C) vehicle or (D) OXA. The eosinophilic microabscess characteristic of this model (and human EoE) is shown in (E) as well as (F) where a microabscess has erupted through the squamous epithelial barrier of the oesophagus. (G) A quantitative assessment of the oesophageal eosinophil infiltrate in each group of mice was determined across nine high powered field (hpf) of view (three distal, three mid and three proximal) and expressed as group mean data of the eosinophil density (ie, eosinophils/400× hpf)±SEM. Scale bar represents 100 μM. These data are representative of three separate experiments with five to nine individual mice per group. *p≤0.05, **p≤0.01. ***p≤0.001. p Values not linked with brackets indicate significance relative to L2-IL5 mice treated with vehicle alone. This figure is only reproduced in colour in the online version.
The spatial pattern of the observed eosinophilia occurring in OXA-treated L2-IL5 mice was also particularly noteworthy because of its similar pattern to what has been described in EoE patients. In particular, a dense epithelial and lamina propria eosinophilia accompanied by eosinophil degranulation occurred in L2-IL5 mice that appeared more at the surface relative to the underlying subepithelial layers (figure 5D). In addition, eosinophilic microabscess formation was evident (figure 5E), which appeared to erupt through the epithelium into the oesophageal lumen (figure 5F).
Oesophageal inflammation occurring in OXA-treated L2-IL5 mice is characterised by cell proliferation of the squamous epithelial basal zone
Squamous epithelial hyperplasia, a key feature associated with human EoE, was demonstrated to occur as part of the eosinophil-dominated inflammation in OXA-treated L2-IL5 mice. Basal zone epithelial cell proliferation, measured by Ki67 immunohistochemistry, was significantly increased in OXA-treated L2-IL5 mice relative to wild-type control mice treated with either vehicle alone or OXA as well as L2-IL5 mice treated with vehicle alone (figure 6A–D). Quantification of Ki67+ cell numbers as the mean of nine or more 400× hpf derived from five to 10 mice per group demonstrated that the squamous epithelial proliferation observed in OXA-treated L2-IL5 mice was statistically higher than that observed in L2-IL5 animals treated with vehicle alone (figure 6E). Increases in epithelial proliferation were not observed comparing vehicle alone versus OXA-treated wild-type animals with both these groups displaying the same level of proliferation as observed in L2-IL5 mice treated with vehicle alone (figure 6E). Similar to human EoE, a gradient of proliferation within the squamous epithelium (ie, localised hyperplasia) was uniquely observed in the oesophagus of OXA-treated L2-IL5 mice. In particular, those epithelial cells in contact with the oesophageal basement membrane (basal zone) differentially stained as Ki67+, encompassing 50–75% of the total epithelial thickness of OXA-treated L2-IL5 mice (figure 6F). In addition, increased proliferation of cells localised within the lamina propria could be observed (figure 6D).
Figure 6.
4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) sensitisation and topical oesophageal challenge of L2-IL5 mice results in squamous epithelial changes indicative of inflammatory events similar to those observed in human subjects with eosinophilic oesophagitis (EoE). Epithelial cell proliferation and expansion of this compartment was determined by immunohistochemistry assessing for the presence of the marker Ki67. Stained oesophageal sections were evaluated and representative photomicrographs are presented from wild-type animals treated either with (A) vehicle or (B) OXA and L2-IL5 mice treated either with (C) vehicle or (D) OXA. (E) The density of Ki67+ cells in the epithelial layer was determined as the mean value (evaluating nine high powered fields of oesophagi (three distal, three mid and three proximal) recovered from 5–10 mice)±SEM. (F) The peak level of basal zone hyperplasia in each group of mice was determined using an algorithm described in the supplementary materials (available online only) and expressed as group mean data ±SEM. Scale bar represents 100 μM. These data are representative of three separate experiments with five to nine individual mice per group. **p≤0.01, ***p≤0.001. This figure is only reproduced in colour in the online version.
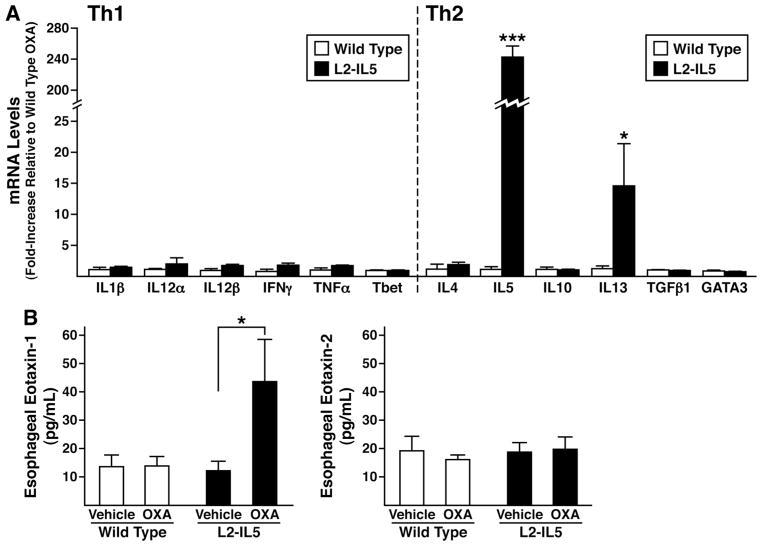
Increases in oesophageal IL-5, IL-13 and eotaxin-1 levels characterised the immune microenvironment of OXA-treated L2-IL5 mice
Gene expression in the oesophagi of OXA-treated L2-IL5 mice was assessed to determine the induced polarisation of the immune microenvironment of this model system. RT–PCR assessments of targeted Th1 versus Th2 cytokine gene expression revealed that the oesophagi of OXA-treated L2-IL5 mice differentially expressed IL-5 and IL-13 (Th2 cytokines) relative to the Th1 cytokine proflle, which remained unchanged (figure 7A). Moreover, oesophageal explant cultures derived from OXA-treated L2-IL5 mice were shown to secrete significantly higher levels of eotaxin-1 (but not eotaxin 2) compared to explants of either wild-type vehicle-alone or OXA-treated animals as well as explants derived from vehicle-alone-treated L2-IL5 mice (figure 7B).
Figure 7.
The induced eosinophilic oesophageal inflammation occurring in 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA)-treated L2-IL5 mice is characterised by a T-helper cell (Th) type 2 predominated cytokine expression profile accompanied by overexpression of the eosinophil agonist chemokine eotaxin-1 but not eotaxin-2. (A) Total RNA derived from whole oesophagi of OXA-treated wild-type versus L2-IL5 mice were used as the starting templates for Taqman based real-time reverse-transcription PCR assessments of expression from genes encoding cytokines characteristic of Th1 versus Th2 immune responses. Expression levels were normalised within each group relative to the levels of 18S ribosomal RNA before determination of fold increase relative to observed levels in the oesophagi of OXA-treated wild-type mice. Individual values are expressed as group means±SEM *p≤0.05, ***p≤0.001. (B) Eotaxin-1 and 2 expression levels derived from the oesophagi of each group of mice were determined by ELISA of supernatants derived from explants cultured for 24 h. These data are representative of two separate experiments and are shown as mean values±SEM of three to five individual mice per group. *p≤0.05, ***p≤0.001.
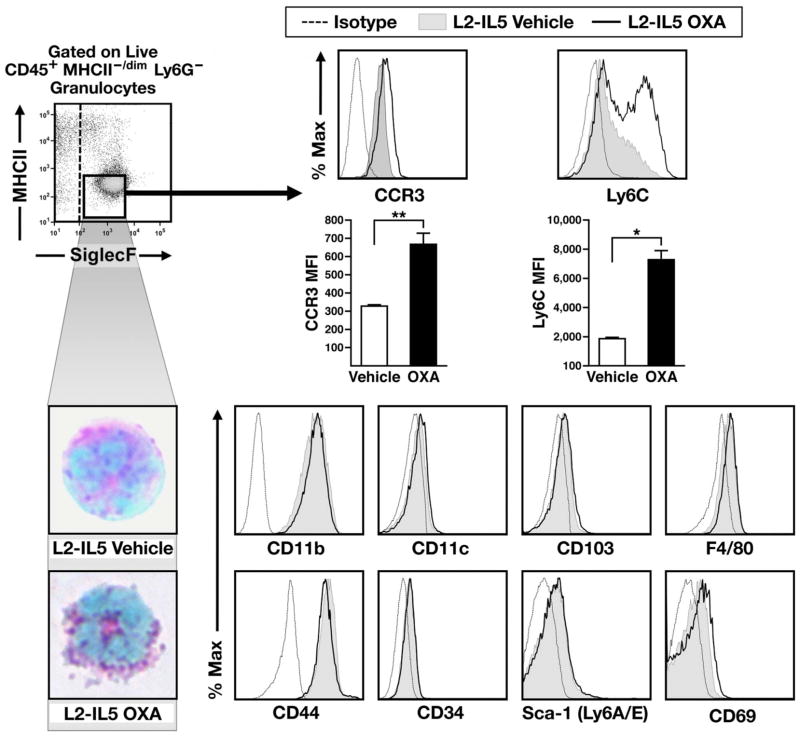
Inflltrating oesophageal eosinophils from OXA-treated L2-IL5 display cell surface phenotypes characteristic of mature tissue resident granulocytes
Oesophageal eosinophils from OXA-treated L2-IL5 mice were compared by flow cytometry relative to eosinophils derived from control L2-IL5 animals treated with vehicle alone. Eosinophils were defined based on a granulocyte scatter-based gate and a surface profile of CD45+, Ly6G−, MHCII−/dim and SiglecF+ (figure 8). Haematological staining and cell differential analysis of these sorted populations confirmed the identity of these cells as mature granule protein-rich eosinophils. Resident oesophageal eosinophils from OXA-treated L2-IL5 mice showed substantially increased expression of cell surface markers CCR3 and Ly6C relative to eosinophils from oesophagi of L2-IL5 mice treated with vehicle alone (figure 8). However, no changes were observed in levels of the other surface markers assessed, including CD11b, CD11c, CD103, F4/80, CD44, CD34, Sca-1 (Ly6A/E) and CD69 (figure 8); IL-5Rα remained close to undetectable in both eosinophil populations (data not shown).
Figure 8.
Infiltrating oesophageal eosinophils from oxazolone in 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA)-treated L2-IL5 display a cell surface phenotype characteristic of mature tissue resident granulocytes. Oesophageal eosinophils are more mature following OXA exposure. (A) Flow cytometric analysis of eosinophils (granulocyte gated, CD45+, Ly6G−, MHCII−/dim, SiglecF+ cells) in vehicle and OXA-treated L2-IL-5 oesophagi. Surface markers CCR3 and Ly6C are increased with OXA challenge. Common markers with other myeloid populations are present in equal abundance in both uninflamed and inflamed oesophagi. (B) These cells were subsequently subjected to fluorescence activated cell-sorting and eventually cell differential analysis of DiffQuick (commercial Romanowsky) stained cytospin preparations of the sorted cell populations, identifying eosinophils in each of these compartments/tissues. Data are representative of two separate experiments, with means±SEM of three to four individual mice per group shown, *p≤0.05, **p≤0.01. This figure is only reproduced in colour in the online version.
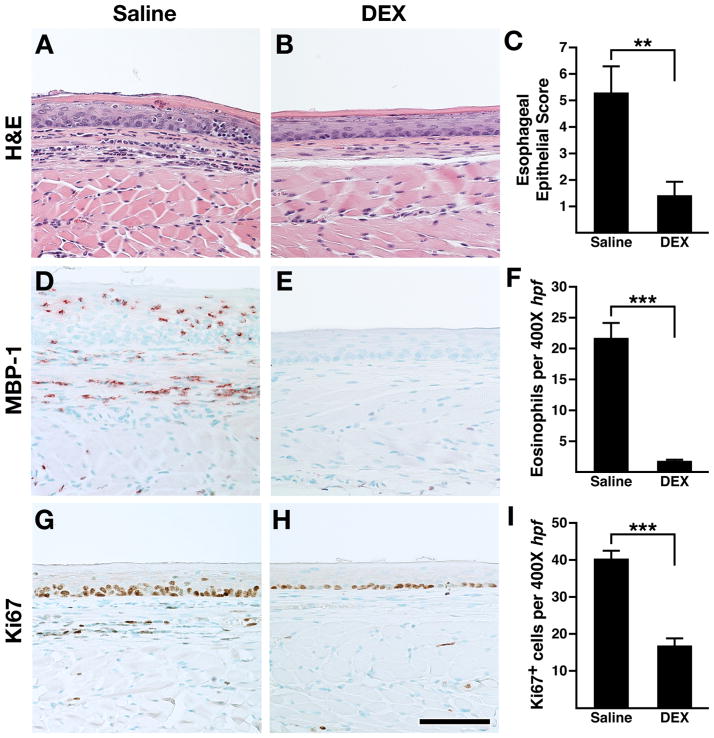
Oesophageal inflammation in OXA-treated L2-IL5 mice was steroid responsive
The successful treatment of EoE patients with corticosteroids1,28 led us to determine if the eosinophilic oesophageal inflammation of the OXA-treated L2-IL5 model of oesophagitis was attenuated by corticosteroid administration. A dexamethasone administration protocol was overlaid onto the OXA treatment timeline, delivering dexamethasone by intraperitoneal injection (vehicle alone served as a negative control) throughout the OXA challenge phase of this inflammatory model (figure 2). Those studies demonstrated that administration of dexamethasone significantly reduced epithelial inflammation and eosinophilia in OXA-treated L2-IL5 mice (figure 9). In particular, H&E stained sections (figure 9A) revealed that dexamethasone administration significantly reduced the proinflammatory cell infiltrate and tissue oedema associated with this model; quantification of this epithelial inflammation demonstrated the significance of this corticosteroid-mediated decrease (figure 9B,C). Immunohistochemical detection of tissue infiltrating eosinophils (ie, MBP-1+ leucocytes) also showed that oesophagi of OXA-treated L2-IL5 mice had evidence of a dense eosinophil infiltrate (figure 9D,E) that was significantly reduced with dexamethasone administration compared to L2-IL5 mice administered vehicle alone (figure 9F). Finally, assessments of squamous epithelial hyperplasia by Ki67 immunohistochemistry showed that dexamethasone treatment led to a statistically significant decline in the expanded proliferating basal zone characteristic of this model (figure 9G,H,I).
Figure 9.
Corticosteroid treatment of in 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (OXA) sensitised/topical challenged L2-IL5 mice leads to the attenuation of all histopathological metrics associated with this mouse model of human eosinophilic oesophagitis (EoE). H&E stained sections of oesophagus (A, B) as well as sections subjected to major basic protein (MBP)-1 (D, E) and Ki67 (G, H) immunohistochemistry were evaluated and representative photomicrographs are presented from OXA-treated L2-IL5 mice treated with either vehicle alone (saline) or dexamethasone (DEX). Dexamethasone was administered to individual mice on protocol day 5, 8, 10 and 12 by intraperitoneal injection of 200 μg dexamethasone (Vedco, St Joseph, Missouri, USA) suspended in 200 μl of saline (figure 2). Control mice received 200 μl of saline alone. (C) The peak level of inflammation (epithelial histopathology score) in each group of mice was determined using an algorithm described in the supplementary materials (available online only) and expressed as group mean data±SEM. (F) A quantitative assessment of the oesophageal eosinophil infiltrate in each group of mice was determined and expressed as group mean data of the eosinophil density (ie, eosinophils/400× high powered field (hpf) (evaluating nine hpf/ oesophagus; three distal, three mid and three proximal))±SEM. (I) The density of Ki67+ cells were determined as the mean value (evaluating nine hpf/oesophagus; three distal, three mid and three proximal)±SEM. Scale bar represents 100 μM. Data are expressed as means±SEM derived from six to 10 individual mice per group. **p≤0.01, ***p≤0.001. This figure is only reproduced in colour in the online version.
DISCUSSION
Clinical studies and therapeutic trials investigating EoE patients have documented a link between this disease and eosinophils. Indeed, these investigations led to a consensus regarding the definition of clinical and pathological descriptions of EoE, diagnostic criteria and treatment guidelines for patients.1,28 The development of animal models and translational studies to identify eosinophil-specific mechanisms eliciting, and/or contributing to, disease pathologies has led to key findings regarding the pathogenesis and therapeutic targets. Mouse models of eosinophilic diseases have been particularly valuable because of the availability of eosinophil-detecting reagents (eg, mouse-specific antibodies), the availability of targeted genetic aberrations (eg, transgenic overexpression and ablation (knockouts) of specific genes of interest), and the ability to perform complete assessment of endpoint pathologies such as measures of physiological parameters, remodelling and postmortem histopathology.18,21,25,29–31 Therefore, together with the increasing understanding of immune responses in the mouse and the realisation of how similar these immune responses are with events occurring in patients, these models have provided needed insights into human EoE, including the mechanistic importance of IL-5 overexpression5,15,16 and the expression of eotaxins.5,11
Our present work that defines a novel mouse model of EoE are based on a number of studies implicating the importance of IL-5 in the pathogenesis of epithelial inflammation in allergic diseases and specifically EoE. For instance, nasal, respiratory and ocular epithelia secrete IL-5 in mouse models of allergic diseases associated with mucosal eosinophilia.14,32–34 A number of translational studies have also identified the oesophageal epithelium of patients (captured by mucosal pinch biopsies) as a source of localised increased IL-5 expression.14,16,32,35 The source of this cytokine is thought to be infiltrating T cells or eosinophils, but the lack of direct visualisation of all expressing cells does not preclude the activated oesophageal epithelium as an additional contributor to local IL-5 levels.14,36 That is, a number of cells are probably contributing to the Th2 milieu of the oesophagus in EoE patients, and we hypothesised that the ectopic overexpression of IL-5 by oesophageal epithelial cells in L2-IL5 mice replicates this localised exacerbation of Th2 immune responses.
Oesophageal studies in the mouse have bifurcated based on the experimental strategies utilised to generate these models of EoE: (1) Sensitisation/local challenge approaches in which wild-type mice undergo a primary sensitisation event to allergen (and/or a contact irritant) followed by a secondary topical challenge of the oesophagus with the same agent.3,6,7 (2) Transgenic overexpression in mice of candidate inflammatory genes identified from clinical studies of EoE patients.5,37 Although both of these approaches have provided valuable insights regarding mechanisms of action, these models have not been organ/tissue specific as transgene expression occurs outside the oesophageal compartment (eg, lymphocyte/ small intestinal driven expression of IL-55 or pulmonary-derived IL-13,37 and some features of the histopathology have not been demonstrated as seen in human EoE. The development of the mouse model used in our study targeted the oesophageal mucosa to overexpress IL-5, thus representing a logical extension of results from translational studies that have previously defined the Th2 mileu. Subsequent use of this model with a local challenge led to key histological, molecular and pharmacological features seen in human EoE.
The relative difference in oesophageal pathologies occurring in the transgenic line alone versus transgenic animals subject to the sensitisation/oesophageal challenge model demonstrated the interplay, and ultimately the importance, of concomitant immune responses and eosinophil-mediated activities. In particular, even though squamous epithelial-specific IL-5 expression alone, without sensitisation and challenge, (ie, L2-IL5 mice) induced a significant oesophageal eosinophilia encompassing the length of the oesophagus (ie, relative to wild-type-sensitised/ challenged wild-type mice), this eosinophilia was not accompanied by the density of histopathological changes such as eosinophilic microabscesses characteristic of EoE. Moreover, unlike L2-IL5 mice subjected to sensitisation/oesophageal challenge, the L2-IL5 vehicle or naive mice did not display evidence of dilated intra-epithelial spaces, basal cell hyperplasia and proliferation of cells within the lamina propria. These observations suggest that the presence of oesophageal eosinophils alone is a necessary but not a sufficient event occurring in EoE. This conclusion suggests a parsimonious explanation of patients who have a tissue eosinophilia consistent with EoE yet display either no overt symptoms or symptoms inconsistent with an EoE diagnosis. In addition, the disconnect between co-stimulatory inflammation and oesophageal eosinophilia may also explain the apparent overlap between some patients with gastro-oesophageal reflux disease. That is to say, in some patients, acid-induced oesophageal inflammation may elicit a tissue eosinophilia without providing the additional needed co-inflammatory signals necessary for the development of EoE symptoms.
The validation that this compound model system is reflective of human EoE was also demonstrated by both the oesophageal expression of other significant inflammatory pathways that have been linked with EoE in clinical and translational studies and the steroid responsiveness of the induced pathologies occurring in OXA-treated L2-IL5 mice. In particular, we demonstrated that this mouse model displayed increased IL-5 expression and the concomitant local expression of IL-13 and eotaxins.11,35 Therefore, unlike sensitisation/oesophageal challenge of the wild-type mice, which elicits only nominal changes in the oesophageal expression of inflammatory markers characteristic of human EoE (ie, IL-13 and/or eotaxins), our compound model of sensitisation/oesophageal challenge of L2-IL5 mice specifically promotes high-level expression of inflammatory markers characteristic of human disease.11,35,38 The observation that this increase was not linked to either IL-5 transgene expression nor the sensitisation/oesophageal challenge alone, but instead was the product of both events, suggests a relationship exists between the robust oesophageal eosinophilia induced in this system and the additional expression of these eosinophilia agonists and/or inflammatory bio-markers characteristic of EoE.
In this current mouse model, we provide a detailed examination of not only the oesophageal mucosa but also the periphery in a consistently ‘primed’ state; IL-5 levels are increased throughout the naive, sensitisation and challenged states in a fashion that may be reflective of the atopic condition EoE. As peripheral eosinophilia may be a surrogate biomarker for therapeutic trials, such as corticosteroids39 and the anti-IL-5 antibody therapy mepolizumab,40,41 this may offer another important model feature. In recent consensus recommendations, it was noted that a subpopulation of EoE patients develop peripheral eosinophilia and this may correlate with tissue eosinophilia; however, these changes should be considered alongside a number of other factors.1 Therefore, while no single systemic biomarker, including peripheral eosinophila, is reliable for EoE, there is a subset of patients for whom the increased levels of systemic eosinophilia found in our model may still be relevant. We anticipate that the complexity of this disease and the limitations of the mouse will require the generation of a number of models like L2-IL5 replicating specific aspects of this disease in an effort to understand EoE mechanistically.
While there are a number of significant similarities between our L2-IL5 model and human EoE, there remain some obvious differences. Examination of the inflammatory response in human EoE includes that of smooth muscle infiltrating mast cell and lamina propria eosinophil production of transforming growth factor β as well as epithelial cell tumour necrosis factor α production, our mouse model of EoE did not. In addition, this model utilises a contact allergen, whereas it is thought patients with EoE develop as an allergic response mainly to food and environmental allergens. As with all animal models, they remain just that, incomplete models of human conditions. Clearly, this is often a shortcoming with virtually all animal models due to immunological, biochemical and physiological species differences. The observations surrounding the L2-IL5 mouse model provide a foundation for a number of future studies that will address specific mechanisms linked with oesophageal remodelling and dysfunction. Whereas this has been addressed in some studies to date, in particular as it pertains to oesophageal fibrosis, additional insights have yet to be gained. For example, the use of food allergens (as opposed to OXA-initiated hypersensitivity) to initiate and perpetuate this response will bring additional relevance to the overall model as it relates to human EoE. Strategic approaches to elicit eosinophil activation/ degranulation in L2-IL5 mice will probably be additional areas of study expected to improve the representative character of this experimental approach of modelling human EoE in the mouse.
The novelty of this mouse model of EoE has been addressed through our reductionist approach of building on observations of patients along with the mechanistic insights and technological expertise of basic research scientists, thus allowing us to move closer to an in-vivo model that closely represents the human disease. We suggest that our creation of a novel model system combining targeted gene expression in the oesophageal epithelia with topical oesophageal challenge of sensitised mice will provide a method with which to define further mechanisms of eosinophil effector function, how these mechanisms integrate with ongoing oesophageal inflammation and the impact of novel therapeutics in these processes. Therefore, we expect that studies of L2-IL5 mice will lead to a greater understanding of the pathophysiological role of eosinophils in barrier dysfunction and remodelling in EoE, with an expectation of valid extrapolation to human patients and the development/ testing of novel therapeutic approaches targeting this localised oesophageal eosinophilia.
Supplementary Material
Significance of this study.
What is already known on this subject?
EoE is an allergic disease characterised by Th2-polarised immune events.
EoE inflammation is characterised by increased numbers of eosinophils, eosinophil microabscess formation and superficial layering of the eosinophils in the oesophageal epithelia.
Therapeutic strategies targeting oesophageal eosinophilia lead to the abatement of this inflammation.
What are the new findings?
The squamous cell-specific promoter EBV-ED-L2 allows for ectopic constitutive expression of heterologous genes in transgenic mice.
Oesophageal epithelial overexpression of IL-5 (L2-IL5) correlates with oesophageal-specific inflammatory events.
Steroid treatment of oxazolone-challenged L2-IL5 mice ameliorates the induced inflammation.
How might it impact on clinical practice in the foreseeable future?
Development of an allergen-naive mouse EoE model that specifically targets the oesophageal epithelia provides a unique and user-friendly preclinical system with which to define underlying immune mechanisms that mediate oesophageal inflammation that can be targeted for therapeutic interventions.
Acknowledgments
The authors wish to thank the members of all the participating laboratories as well as colleagues within the greater eosinophilic oesophagitis community for insightful discussions and critical comments that directly led to the development of this unique transgenic line of mouse and the novel multistrategic approach to create a mouse model representative of human EoE. They also wish to acknowledge the invaluable assistance in animal husbandry and care at University of Colorado Denver (Kristann Magee), the Mayo Clinic Arizona medical graphic artist (Marv Ruona) and the administrative support provided by Linda Mardel and Shirley (‘Charlie’) Kern.
Funding Grants from the USA National Institutes of Health (GTF (R01 DK 62245), JJL a K26 award from the National Center for Research Resources (RR0109709) and HKE (R01-HL0921, R01-DK083385 and R01-HL098294)), Crohn’s and Colitis Foundation of America ( JCM, HKE), the North American Society for Pediatric Gastroenterology Hepatology and Nutrition ( JCM) and the Mayo Foundation for Medical Education and Research ( JJL) as well as gifts from Shell, Mandell, Sovoie and Boyd families were the sources of funding used in the performance of studies including data analysis and manuscript preparation.
Footnotes
Additional supplementary data are published online only. To view these files please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2012-303631).
Contributors: All authors contributed to data generation, data analysis and interpretation as well as writing of this manuscript. JLL and GTF are co-senior authors of this study.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are available for public view.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 2.Masterson JC, Furuta GT, Lee JJ. Update on clinical and immunological features of eosinophilic gastrointestinal diseases. Curr Opin Gastroenterol. 2011;27:515–22. doi: 10.1097/MOG.0b013e32834b314c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akei HS, Mishra A, Blanchard C, et al. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–94. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Mishra A, Hogan SP, Brandt EB, et al. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra A, Hogan SP, Brandt EB, et al. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–9. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 6.Rajavelu P, Rayapudi M, Moffitt M, et al. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G645–54. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinstein E, Cho JY, Rosenthal P, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–16. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonsalves N, Yang GY, Doerfier B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–9. e1. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Spergel JM, Andrews T, Brown-Whitehorn TF, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 10.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–63. 63 e1–3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–19. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 15.Mavi P, Rajavelu P, Rayapudi M, et al. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1347–55. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa H, Wang TC, Zukerberg L, et al. The targeting of the cyclin D1 oncogene by an Epstein–Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–90. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- 18.Lee NA, McGarry MP, Larson KA, et al. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–44. [PubMed] [Google Scholar]
- 19.Nakagawa H, Inomoto T, Rustgi AK. A CACCC box-like cis-regulatory element of the Epstein–Barr virus ED-L2 promoter interacts with a novel transcriptional factor in tissue-specific squamous epithelia. J Biol Chem. 1997;272:16688–99. doi: 10.1074/jbc.272.26.16688. [DOI] [PubMed] [Google Scholar]
- 20.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 21.Masterson JC, McNamee EN, Jedlicka P, et al. CCR3 blockade attenuates eosinophilic ileitis and associated remodeling. Am J Pathol. 2011;179:2302–14. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamee EN, Wermers JD, Masterson JC, et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm Bowel Dis. 2010;16:743–52. doi: 10.1002/ibd.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108:16711–16. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamee EN, Masterson JC, Jedlicka P, et al. Ectopic lymphoid tissue alters the chemokine gradient, increases lymphocyte retention and exacerbates murine ileitis. Gut. doi: 10.1136/gutjnl-2011-301272. Published Online First: 20 Jan 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochkur SI, Jacobsen EA, Protheroe CA, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 26.Boirivant M, Fuss IJ, Chu A, et al. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–39. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Justice JP, Borchers MT, Crosby JR, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–78. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 31.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya B, Carlsten J, Sabo E, et al. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol. 2007;38:1744–53. doi: 10.1016/j.humpath.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Lucendo AJ, De Rezende L, Comas C, et al. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am J Gastroenterol. 2008;103:2184–93. doi: 10.1111/j.1572-0241.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu CA, Peluso JJ, Zhu L, et al. Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cell Immunol. 2010;264:32–41. doi: 10.1016/j.cellimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–17. 17 e1–7. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straumann A, Kristl J, Conus S, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720–6. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 37.Zuo L, Fulkerson PC, Finkelman FD, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185:660–9. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37. 37 e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 41.Conus S, Straumann A, Bettler E, et al. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:175–7. doi: 10.1016/j.jaci.2010.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.