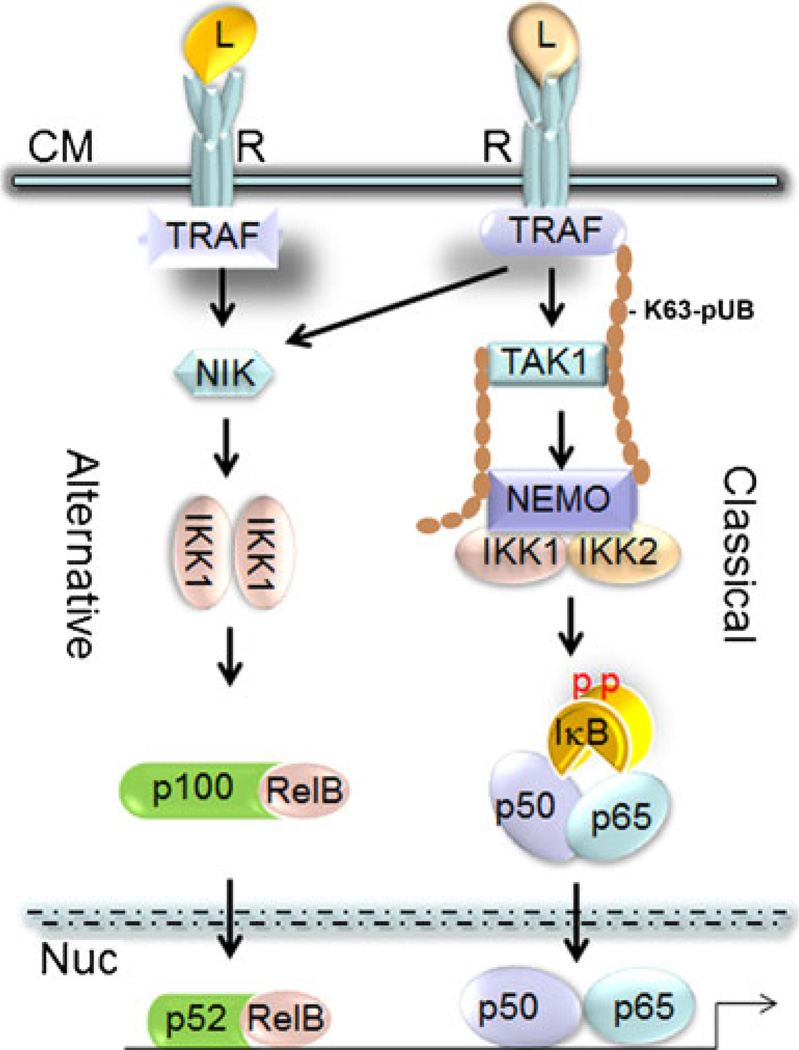
Fig. 1.
Illustration of NF-κB signaling pathway depicting classical and alternative arms. Ligand (L) binding to its cell membrane (CM) receptor (R) initiates recruitment and formation of a signaling cluster at the distal end of the receptor. Signaling complexes contain a large number of proteins including TNF receptor-associated factors (TRAFs), the tyrosine kinase c-Src, p62, cellular inhibitors of apoptosis (c-IAP), and TNF receptor-interacting protein (RIP). This cluster utilizes lysine 63-linked polyubiquitination chains (K63-pUB) to recruit and activate the MAP kinases TGF-β-activated kinase (TAK1) and NF-κB-inducing kinase (NIK) which in turn activate the canonical and alternative IKK complexes, respectively. Activated IKK1 and IKK2 phosphorylate (pp) their respective p100/NF-kB and IkB targets, which are subsequently degraded. Processed p52 along with RelB as well as liberated p50/p65 dimers translocate to the nucleus (Nuc), bind to DNA sequences, and activate transcription. L ligand, R receptor, CM cytoplasmic membrane, Nuc nucleus, p phosphorylation