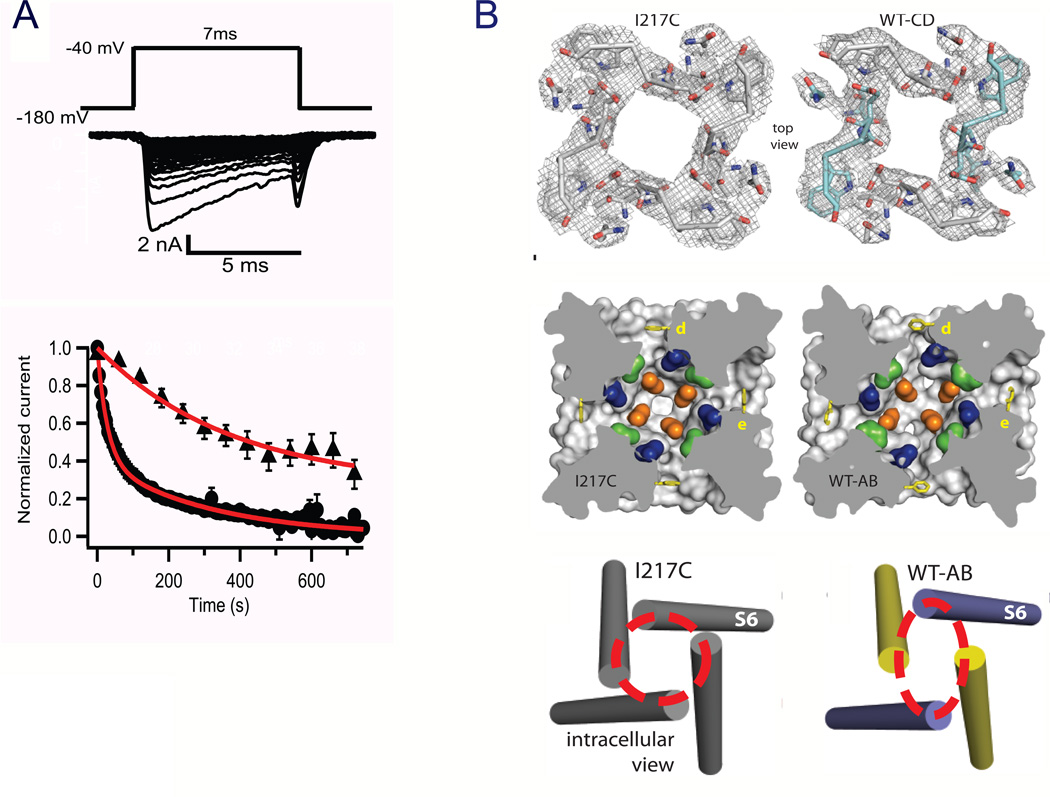
Figure 10. Structure of the slow-inactivated state in NaVAb.
A. Use-dependent development of slow inactivation. Depolarizations from a holding potential of −180 mV to −40 mV, 7 ms in duration, were applied at 0.2 Hz (circles) or once per min (triangles), and the peak current elicited by each pulse was measured. Currents were normalized to the peak inward current during the first pulse. BTop, selectivity filter. Stick representation of the selectivity filter with a 2Fo-Fc map calculated at 3.2 Å resolution (grey mesh) contoured at 1.5 σ for NaVAb -I217C and 1.0 σ for NaVAb -CD. Symmetry-related subunits in WT-CD are colored white (Chains A and D) and cyan (Chains B and C), respectively. Middle, central cavity. A view through the pore module sectioned below the selectivity filter illustrates the lateral pore fenestrations, hydrophobic access to the central cavity, and structural asymmetry in the NaVAb-AB pore domain. Phe203 side-chains are yellow sticks. NaVAb residues implicated in drug binding in vertebrate NaV channels are colored: Thr206 (blue), Met209 (green), and Val213 (orange). Bottom, activation gate. Red dashed lines indicate the Cα location of D219 (the last S6 residue modeled in WT-AB), where the S6 helices are shown as cylinders. WT-chain A, purple; WT-chain B, yellow (Payandeh et al., 2012).