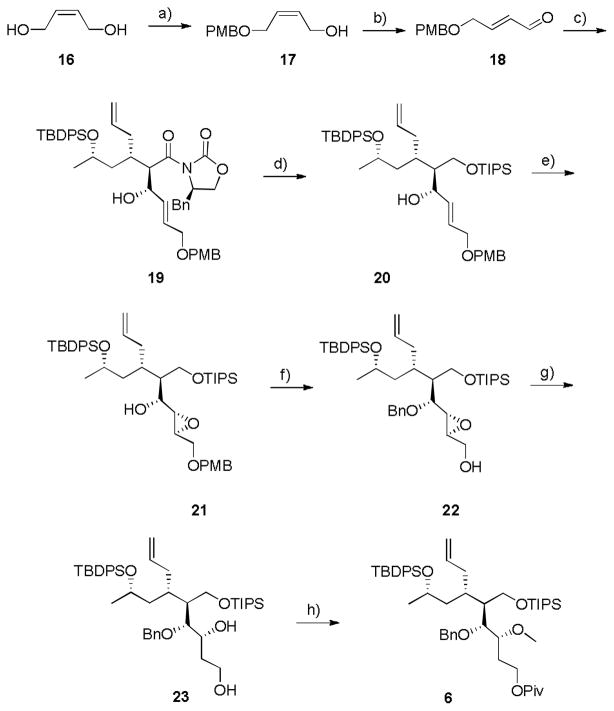
Scheme 3.
Synthesis of key intermediate 6. a) NaH, TBAI, PMBCl, THF, 0 °C, rt, 12 h, 68%; b) MnO2, CH2Cl2, rt, 36 h, 36%; c) i. TiCl4, DIPEA, NMP, CH2Cl2, 0 °C, ii. 18, CH2Cl2, 0 °C, 65% (10:1 dr); d) i. LiBH4, MeOH, THF, 0 °C, 6 h, 44%; ii. TIPSCl, ImH, CH2Cl2, rt, 4 h, 82%; e) VO(acac)2, TBHP, CH2Cl2, 0 °C, 3 h, 95%; f) i. BnBr, NaH, TBAI, THF, 0 °C, 4 h, 85%; ii. DDQ, CH2Cl2, pH 7 buffer, 0 °C, 14 h, 73%; g) Red-Al, THF, 0 °C to rt, 4 h, 79% (>20:1, 1,3:1,2); h) i. PivCl, pyr, CH2Cl2, rt, 3h; ii. Me3OBF4, CH2Cl2, rt, 1.5 hr, 75% over two steps. PMB = para-methoxybenzyl, TBAI, tetrabutyl ammonium iodide, TBDPS = tert-butyldiphenylsilyl, TIPS = triisopropylsilyl, ImH = imidazole, TBHP = tert-butyl hydrogen peroxide, Bn = benzyl, Piv = pivalate, pyr = pyridine.