Abstract
Premature ovarian insufficiency (POI) is a main cause of infertility and affects nearly 1% women under 40 years old. This study was aimed to utilize the side effects of tripterygium glycoside (TG) to induce a mouse model of POI. 48 female KM mice were divided into four groups: control, oral, intraperitoneal injection and subcutaneous injection group. The mice in last three groups were treated with TG (50 mg•kg-1) daily for 35 days, while the mice in control group were treated with parallel volume of sterile water. Vaginal smears were taken to monitor the estrous cycles and estrous frequency for the last 21 days. Ovarian and uterine index and histomorphological change were determined when finished the administration. Serum levels of FSH were assessed by ELISA. Ki-67 expression in the uterus was analyzed from using immunohistochemical detection. And the apoptosis of follicle cells were detected by TUNEL assay. The results showed that mice in subcutaneous injection group presented the critical manifestations with significantly prolonged estrous cycles, decreased estrous frequency, reduced ovarian and uterine index, and increased serum FSH levels. At this dose level, TG could reduce developing follicles and corpus luteum, and increase atretic follicles, which might be induced by the increasing levels of follicle apoptosis. The proliferation index of uterus, evaluated by histomorphological changes and the expression of Ki-67, was significantly suppressed in TG treated animals. These data suggested that TG was feasible to induce a mice model via subcutaneous injection which could mimic the manifestations of POI.
Keywords: Premature ovarian insufficiency, mouse model, tripterygium glycoside
Introduction
Premature ovarian Insufficiency (POI) is defined as the cessation of ovarian function before the age of 40, which is characterized by the presence of oligomenorrhea or amenorrhea, elevated gonadotropins, and low estrogen levels. The known etiologies of POI are: genetic defects; iatrogenic damages including surgery, chemotherapy and radiotherapy; autoimmune ovarian damage; infection; environmental toxins such as 4-vinylcyclohexene diepoxide (VCD); metabolic factors and so on [1-4]. However, the cause of most POI patients still remains unknown. Because of long-term deprivation of estrogen in POI patients, various aspects of patients’ heath would be impacted, including fertility, maintenance of bone mass and cardiovascular systems, life-expectancy, cognitive function, sexual function, associated endocrine and autoimmune conditions, etc [3,5-7]. So far, the mechanism, early diagnose and therapy of POI have still been a significant problem, which needs further and deeper research. Due to poor feasibility of human studies, an ideal animal model of POI is in demand. Tripterygium glycoside (TG), a traditional Chinese patent medicine, is widely used to treat autoimmune and inflammatory diseases such as rheumatoid arthritis. However, a long-term use of TG could cause irregular menstruation, amenorrhea and even premature ovarian insufficiency [8]. So side effects of TG on female reproductive system may probably be used to induce an ideal animal model of POI. Based on this, our objective was to take advantage of TG and induce a mouse model with the similar manifestations to POI patients. In this particle, we compared different administration routes to select a better way to induce a new mouse model of POI, and further evaluated various reproductive parameters of female KM mice treated with TG.
Materials and methods
Chemicals
TG was purchased from Huangshi Feiyun Pharmaceutical (10 mg/tab, batch number Z42021212, China). 60 mg TG was dissolved in 1 ml dimethyl sulfoxide (DMSO), and then the mixture was dissolved in 11 ml sterile water to be 5 mg•ml-1 of final concentration. Meanwhile, the equal 1 ml DMSO was added into 11 ml sterile distilled water as placebo.
Animals
Female KM mice (6 weeks of age) were obtained from the Laboratory Animal Center of Fudan University (Shanghai, China). Experimental animals, which were housed in groups of 4 per wire cage, were kept under standard laboratory conditions (12 hours of light, 12 hours of dark; 25°C) for 10 days to acclimatize to laboratory conditions. During acclimatization, standard mouse chow and water were available ad libitum.
Treatment
48 healthy female KM mice were divided into 4 groups: control group (control), oral group (oral), intraperitoneal injection group (ip) and subcutaneous injection group (sc). Mice in oral, ip and sc group were treated with TG 50 mg•kg-1 everyday via different administration routes for 35 days. At the same time, mice in the control group were given parallel volume of sterile water. The day after the last dose, all the mice were anesthetized by intraperitoneal injection with 10% chloral hydrate solution (0.3 ml•100g-1) in diestrus. Body weights of animals were recorded every week and blood samples were collected to determine serum levels of FSH. Ovaries and uteruses were weighted and immediately fixed with 4% paraformaldehyde for 48 hours.
Estrous phase
During the last 21 days, the estrous cycles of the female mice were routinely assessed by vaginal smear. The abnormal rate of estrous cycles and estrous frequency were analyzed.
Serum levels of FSH
Blood samples were centrifuged at 3,000 rpm for 8 minutes to separate serum. Serum was then collected and stored at -80°C until analysis. Serum FSH was measured by enzyme-linked immune sorbent assay (ELISA), using 96-well plates. Mouse FSH ELISA kit was purchased from bio-swamp, China.
Ovarian and uterine index
Ovaries and uteruses were weighted immediately after they were taken out from mice. Then the Ovarian and uterine index were calculated as organ-to-body-weight ratio.
Histomorphology of ovary and uterus
After the ovaries and uteruses were fixed in 4% paraformaldehyde for 2 days, they were embedded in paraffin, serially sectioned at 4 μm, and stained in hematoxylin and eosin for microscopic observation. Then developing follicles and corpus luteum were counted according to the reference [9].
Immunohistochemistry
Briefly, after deparaffinization and rehydration, antigen retrieval was performed by incubating the sections in 0.01 M citrate buffer (pH 6.0) and applying high microwave irradiation for 30 min. The endogen peroxidase activity was inhibited with 3% H2O2 for 10 min and nonspecific binding was blocked with 10% normal goat serum for 1 h. All sections were incubated with the primary antibodies for Ki-67 (1:100, Abcam, UK) for 24 h at 4°C and then were placed for 45 min at room temperature. Biotinylated secondary antibodies were added for 10 min and then all sections were incubated were incubated with horseradish peroxidase (HRP) for 10 min. Visualization of the antigens was achieved by diaminobenzidine (DAB). Finally, the slides were counterstained with hematoxylin, dehydrated and mounted. At the same time, breast cancer sections were used as positive control, and negative control were treated by substituting phosphate buffered saline (PBS) for primary antibodies.
Follicle cell apoptosis detection by TUNEL assay
Apoptotic cells in ovarian tissue sections were identified by in situ cell death detection kit (Roche Company, Germany). The main procedures were as follows: (a) Deparaffinization and rehydration: all the slides were immersed in xylene for 10 min × 2 times, then 100-70% gradient concentration of alcohol for 5 min each, followed by PBS for 5 min × 2 times. (b) Permeabilization: all the slides were incubated in the freshly prepared 0.1% Triton X-100 permeabilization solution with 0.1% citrate buffer was added for 8 min, and then washed by PBS. (c) Immerse the slides for 30 min at room temperature in Tris-HCl, 0.1 M pH 7.5, containing 3% BSA and 20% normal bovine serum, then rinse the slides twice with PBS. (d) Add 50 μL of TUNEL reaction mixture on the section and incubate for 1 h at 37°C. (e) Rinse the slides and evaluate the section under a fluorescence microscope.
Statistical analysis
The data was presented as mean ± standard deviation. The difference in body weight, estrous frequency, organ index, serum FSH level, the number of follicles and corpus luteum were compared by independent sample t-test by SPSS software (version 16.0). The abnormal rate of estrous cycles was compared by chi-square test. A P value <0.05 was defined as statistically significant.
Results
The manifestations of mice treated with TG via different administration routes
No apparent toxicity was observed in all groups. TG had an important influence on estrous cycles in female mice. It prolonged the length of estrous cycles significantly. The abnormal rate of estrous cycles was obviously high in the mice treated by TG (Figure 1A), and the number of estrous frequency per animal was significant reduced, especially in ip and sc groups when compared to control animals (Figure 1B). Additionally, contracted ovaries and uteruses were observed obviously in ip and sc groups, which were ensured by reduced relative weight of ovary and uterus (Figure 1C, 1D). In Figure 2, different stages of follicles and distinguished corpus luteum were clearly observed in control group. TG treated could induce ovarian fibrosis seriously, and follicle development was suppressed, with thinner layers of granulosa cells and more degenerative and vacuolar changes. The phenomena were distinctly in sc group, with rare corpus luteum, reduced follicles, and serious fibrillation.
Figure 1.
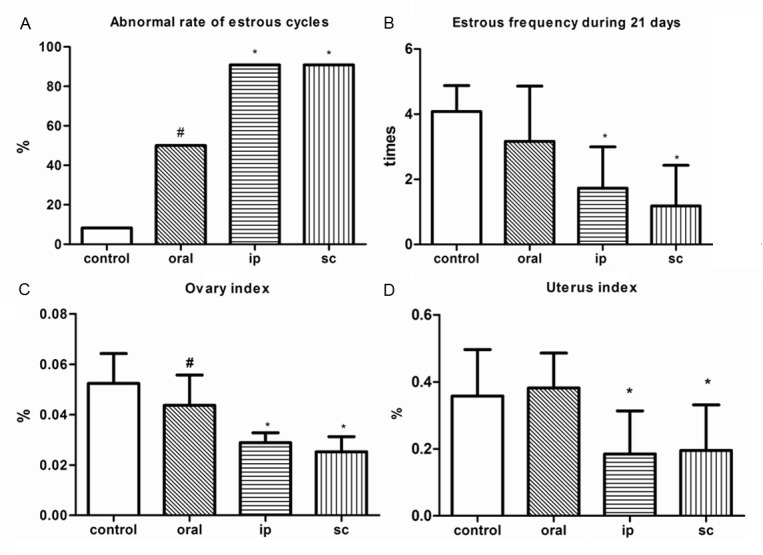
The comparison of manifestations among different administration route. #: P<0.05, compared with control group; *: P<0.01, compared with control group.
Figure 2.
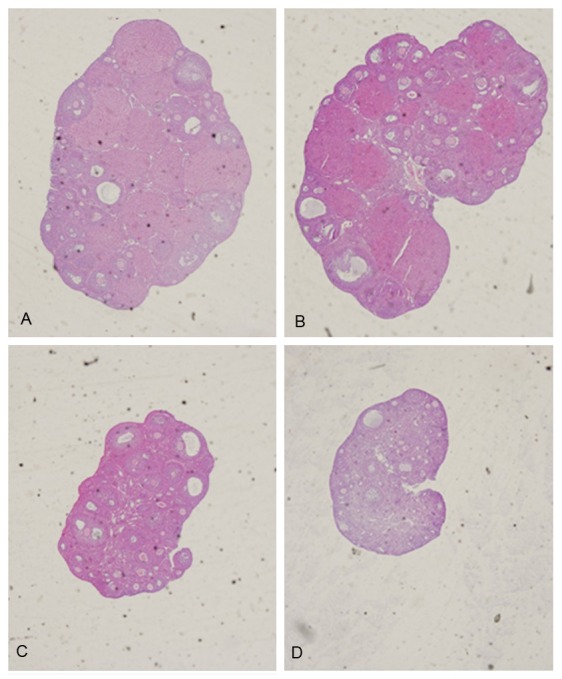
The changes of ovarian histomorphology in 4 groups. A: Control group; B: Oral group; C: Ip group; D: Sc group. (HE × 40).
Mice in sc group showed the manifestations resembling POI patients
As presented in Table 1, the growth condition of mice in sc group didn’t show any difference and body weight gains were parallel, when compared with control group. The abno-rmal rate of estrous cycles was obviously high in sc group, and the number of estrous frequency per animal was significantly red-uced. Ovarian and uterine index were distinctly reduced and serum FSH levels were increased in sc group. The changes of ovarian histomorphology were obvious with reduced developing follicles and corpus luteum, when compared with control group. All these manifestations in sc group resembled the feature of POI.
Table 1.
The manifestations between sc and control group (χ̅ ± S, n=12)
| Control | Sc | ||
|---|---|---|---|
| Body weight before administration (g) | 25.86 ± 1.33 | 26.05 ± 1.52 | |
| Body weight after administration (g) | 34.64 ± 2.40 | 34.11 ± 2.36 | |
| Abnormal rate of estrous cycles (%) | 8.33 | 90.91 | * |
| Estrous frequency | 4.08 ± 0.79 | 1.18 ± 1.25 | * |
| Ovarian index (%) | 0.052 ± 0.011 | 0.025 ± 0.006 | * |
| Uterine index (%) | 0.357 ± 0.139 | 0.195 ± 0.136 | * |
| FSH (U/L) | 7.43 ± 2.37 | 10.55 ± 2.31 | * |
| Follicles number | 37.92 ± 10.84 | 19.82 ± 6.38 | * |
| Corpus luteum number | 10.42 ± 4.52 | 1.09 ± 1.92 | * |
P<0.01, compared with control group.
TG treatment inhibited uter-us proliferation
In macroscopic view, uterine was significantly contracted in sc group (Figure 3A, 3B), in accordance with the index showed above. Add-itionally, from a microscopic view, the histomorphology of the uterus also revealed that treatment with TG could cause a disorganization of the uterine epithelial cells, partially presenting nuclear fragmentation and even reduced in number (Figure 3C), when compared with control group (Figure 3D). Obviously, the endometrium of uterus was thinner than control group, and the glands were specifically reduced. The proliferation index of uterus was evaluated by Ki-67, which was expression at the cubic epithelia cells of uterine endometrium and glands. Both of the uterine epithelial cells and glands presented lower expression of Ki-67, when compared to control group (Figure 4).
Figure 3.
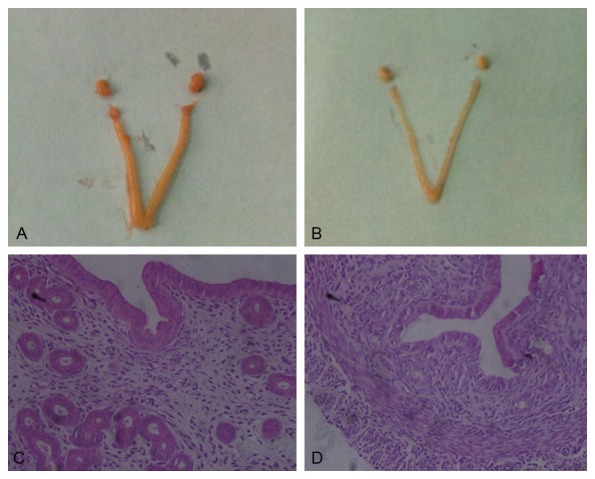
Effects of TG on macroscopic and microscopic feature of uterine. A and C: Control group; B and D: Sc group; C and D: HE × 400.
Figure 4.
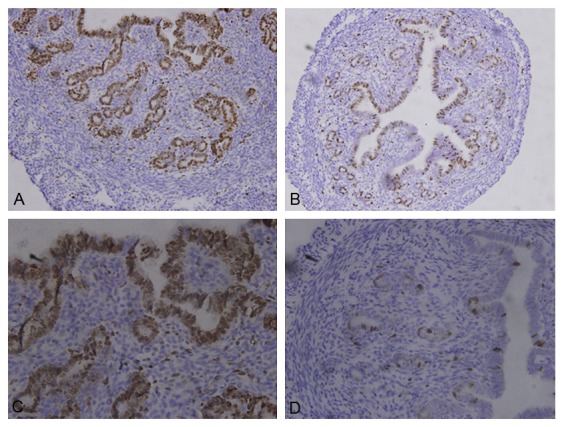
Location of Ki-67 by immunohistochemistry. A and C: Control group; B and D: Sc group. A and B: × 200; C and D: × 400. Weak Ki-67 staining was observed in sc group.
TG treatment induced follicle cell apoptosis
There were few TUNEL-positive follicle cells in the section from control group. By contrast, the number of TUNEL-positive follicle cells inc-reased obviously in TG treated mice. Repeated researches also revealed that there were significantly increased apoptotic rate of developing follicles in TG treated mice, selective images were show in Figure 5.
Figure 5.
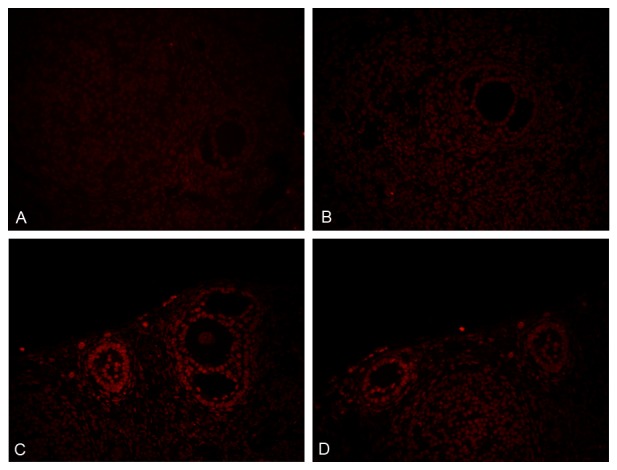
Detection of follicle cell apoptosis by TUNEL assay (× 400). A and B: Control group, C and D: Sc group.
Discussion
Premature ovarian failure (POF), premature menopause and primary ovarian insufficiency are frequently-used terms to define deviation from healthy ovarian function. As ovarian insufficiency describes a wide range of impaired ovarian function and patients may not experience the end of ovarian function at the time of diagnosis and may intermittently recover [3,10], we regard premature ovarian insufficiency as more accurate than premature ovarian failure. POI is a main cause of infertility and affects nearly 1% women under 40 years old [11]. As increasing numbers of women suffers from POI, diminished ovarian function and infertility is of concern.
For the sake of poor feasibility of human studies, it is critical to have ideal models that mimic events occurring among patients who are suffering POI. Mouse knockout models have been used to detect the genes which are associated with the development and apoptosis of follicles and subsequent damage and exhaustion of the follicle pool leading to POI [12,13]. And autoimmune premature ovarian insufficiency models and chemotherapy-induced models have also been used to study the treatment of autoimmune ovarian damage and chemotherapy-induced POI [14-16]. Though, the drug-induced POI has received relatively little attention. The ad-verse effects of Tripterygium Wilfordii Hook F (TWHF, also known as Lei Gong Teng) and its preparations such as TG on the female reproductive system draw our attention to the possibility of establishing a new POI model by the treatment of these medicines. TG induced POI rat model was applied in many researches carried out by Chinese scholars [17]. However, the mechanism of TG acting on reproductive systems was unknown, the characteristics of TG induced models were not presented in detail, and the way to establish the model was not surely defined. During our researches, different administration routes of TG treatment were compared. Combining the presentation of estrous cycles, organ index and ovarian histomorphology, it is feasible to establish mouse model of premature ovarian insufficiency with TG, and we found that the effects on the reproductive systems induced via subcutaneous injection were better than traditional oral administration.
So this article shows the mouse model of POI utilizing the reproductive toxicity of TG and estimates various reproductive parameters in TG treated mice. Estrous phase, the external indicator of gonadal activities, was carefully recorded. And any physiological and histomorphological changes within the ovaries could induce the abnormality of estrous cycles. Disordered manifestations included prolonged duration of es-trous cycles and continuous diestrus in TG treated mice. And it was confirmed that TG treatment could significantly suppressed estrous frequency, which was consistent with the ovulation suppression showed with the numbers of corpus luteum. The higher level of FSH might be the result of damage of ovarian function. Contracted ovaries and uterus were clearly observed in TG treated mice and the index was decreased greatly, which also refl-ected the toxicity on ovarian functions. Further, accounting of ovarian follicles revealed that TG treatment could significantly reduce developing follicles and incr-ease atretic follicles in the ovaries, which may be due to the increase of apoptosis of follicle cells. On the other hand, TG treatment induced pathological changes of uterine endometrium and glands. Combined with the detection of the expression of Ki-67 in uterus, we may deduce that TG inhibited the proliferation of uterine endometrium and glands, which was against to fertilize.
The approach of administration of TG via subcutaneous injection was first attempted. And as above, TG treated mice displayed abnormal estrous cycles, decreased estrous frequency, high level of FSH, decreased ovarian and uterine index, reduced developing follicles and corpus luteum, and increased atretic follicles. And the probably mechanism involved in TG induced POI mouse model may be the detection of follicle cells apoptosis and the follicular maturation blocking proved by decreased corpus leutum. And the histomorphologic changes and Ki-67 expression of uterus supplemented the impacts on uterus by TG treatment.
As the diverse etiology of POI, various POI models should be established to cover the whole spectrum of the disease and help to clarify the pathogenesis and develop novel therapeutic methods. We choose KM mice for their short reproductive life spans, high reproduction index, stable genetic backgrounds, and low costs. TG is commonly used in clinic and convenient to obtain, and the method of establishing the model was easy to master. What’s more, TG treated mice displayed paralleled manifestations to clinic features of POI patients. In conclusion, this research is helpful to complete the animal models of POI and TG induced POI model may be beneficial to pave the way for the researches on prevention and treatment of POI. Certainly, if controlled release formulations of TG could be manufactured, the injury followed by subcutaneous injection was reduced. And if the targeting drug delivery system could be realized, the multiple pharmacological activities on other system were also reduced. That’s an open research question.
Acknowledgements
All the experiments were accomplished in Key Laboratory of Molecular Medicine, Ministry of Education, Department of Biochemistry and Molecular Biology, Institute of Medical Sciences, Shanghai Medical College, Fudan University. And this research was supported by the Shanghai Municipal Committee of Science and Technology Project (10140901501).
Disclosure of conflict of interest
None.
References
- 1.Gannon AM, Stampfli MR, Foster WG. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci. 2012;125:274–284. doi: 10.1093/toxsci/kfr279. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimi M, Asbagh FA. Pathogenesis and causes of premature ovarian failure: an update. Int J Fertil Steril. 2011;5:54–65. [PMC free article] [PubMed] [Google Scholar]
- 3.De Vos M, Devroey P, Fauser B. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 4.Haas JR, Christian PJ, Hoyer PB. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med. 2007;57:443–449. [PubMed] [Google Scholar]
- 5.Wellons M. Cardiovascular disease and primary ovarian insufficiency. Semin Reprod Med. 2011;29:328–341. doi: 10.1055/s-0031-1280918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarante F, Vilodre LC, Maturana MA, Spritzer PM. Women with primary ovarian insufficiency have lower bone mineral density. Braz J Med Biol Res. 2011;44:78–83. doi: 10.1590/s0100-879x2010007500122. [DOI] [PubMed] [Google Scholar]
- 7.Maclaran KE, Horner E, Panay N. Premature ovarian failure: long-term sequelae. Menopause Int. 2010;16:38–41. doi: 10.1258/mi.2010.010014. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Chen SL. A woman with premature ovarian failure induced by Tripterygium wilfordii Hook. f. gives birth to a healthy child. Fertil Steril. 2011;96:19–21. doi: 10.1016/j.fertnstert.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–80. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 10.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 11.Goswami D, Conway GS. Premature ovarian failure. Horm Res. 2007;68:196–202. doi: 10.1159/000102537. [DOI] [PubMed] [Google Scholar]
- 12.Bouhali K, Dipietromaria A, Fontaine A, Caburet S, Barbieri O, Bellessort B, Fellous M, Veitia RA, Levi G. Allelic reduction of Dlx5 and Dlx6 results in early follicular depletion: a new mouse model of primary ovarian insufficiency. Hum Mol Genet. 2011;20:2642–2650. doi: 10.1093/hmg/ddr166. [DOI] [PubMed] [Google Scholar]
- 13.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T, Huang Y, Guo L, Cheng W, Zou G. CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int J Med Sci. 2012;9:592–602. doi: 10.7150/ijms.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghadami M, El-Demerdash E, Zhang D, Salama SA, Binhazim AA, Archibong AE, Chen X, Ballard BR, Sairam MR, Al-Hendy A. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012;7:e32462. doi: 10.1371/journal.pone.0032462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang CL, Li F, Sun L, Li DJ. Therapeutic effect of bushen huoxue recipe on autoimmune premature ovarian failure mice established by immunization with recombinant porcine zona pellucida 4 antigen. Chin J Integr Med. 2013;19:439–445. doi: 10.1007/s11655-012-1025-y. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y, Zhao Z, Wu Y, Wu K, Xu X, Liu Y, Tong C. Therapeutic mechanisms of Tongmai Dasheng Tablet on tripterygium glycosides induced rat model for premature ovarian failure. J Ethnopharmacol. 2012;139:26–33. doi: 10.1016/j.jep.2011.08.077. [DOI] [PubMed] [Google Scholar]


