Abstract
Regional cortical thickness alterations have been reported in many chronic inflammatory and painful conditions, including inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS), even though the mechanisms underlying such neuroplastic changes remain poorly understood. In order to better understand the mechanisms contributing to grey matter changes, the current study sought to identify the differences in regional alterations in cortical thickness between healthy controls and two chronic visceral pain syndromes, with and without chronic gut inflammation. 41 healthy controls, 11 IBS subjects with diarrhea, and 16 subjects with ulcerative colitis (UC) underwent high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo scans. Structural image preprocessing and cortical thickness analysis within the region of interests were performed by using the Laboratory of Neuroimaging Pipeline. Group differences were determined using the general linear model and linear contrast analysis. The two disease groups differed significantly in several cortical regions. UC subjects showed greater cortical thickness in anterior cingulate cortical subregions, and in primary somatosensory cortex compared with both IBS and healthy subjects. Compared with healthy subjects, UC subjects showed lower cortical thickness in orbitofrontal cortex and in mid and posterior insula, while IBS subjects showed lower cortical thickness in the anterior insula. Large effects of correlations between symptom duration and thickness in the orbitofrontal cortex and postcentral gyrus were only observed in UC subjects. The findings suggest that the mechanisms underlying the observed gray matter changes in UC subjects represent a consequence of peripheral inflammation, while in IBS subjects central mechanisms may play a primary role.
Introduction
Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) are characterized by chronically recurring symptoms of abdominal pain associated with flares of mucosal inflammation. In contrast, in irritable bowel syndrome (IBS), chronically recurring symptoms of abdominal pain and discomfort occur in the absence of mucosal inflammation or other identifiable nociceptive triggers (therefore referred to as “functional” pain syndromes), and symptom flares are often triggered by psychosocial stressors. It is generally assumed that abdominal pain in UC results initially from inflammation induced peripheral and central sensitization of visceral afferent pathways [1], while symptoms in IBS may reflect primarily an alteration in central pain modulation, including alterations in endogenous descending pain modulation mechanisms [2]. On the other hand, several pieces of evidence support the concept that IBD patients effectively engage endogenous pain inhibition systems [3], including greater engagement of a cortico limbic-pontine pain modulation network compared to IBS subjects [4]. These differences in the engagement of endogenous pain modulation systems may explain the clinical observation that in uncomplicated UC, abdominal pain is not a prominent symptom even during flares.
Several studies have applied multimodal brain imaging to investigate the presence of grey matter changes in patients with various chronic pain conditions without known nociceptive drive [5]–[9], with presumed nociceptive drive [10]–[12], and with known inflammatory drive [13]–[18]. Reported abnormalities in these studies suggest some similarities in findings (e.g. gray matter reduction in insula [INS] and anterior cingulate cortex [ACC] subregions), and increases in CT in somatosensory regions regardless of pain syndrome, and no clear differences have emerged between the different pain categories, in particular between chronic visceral pain of “functional” and of inflammatory origin. The use of different analysis techniques by different groups, makes interpretive comparisons between studies and between different patient populations more difficult.
In the current study, we used the Laboratory of Neuroimaging (LONI) Pipeline [19], [20] for image preprocessing, volumetric analysis and cortical thickness (CT) analysis. We focused on differences of local morphologic brain alterations between UC and healthy control subjects (HCs), and compared them to findings in IBS subjects. Specifically, we aimed to test the following hypotheses: 1) Both IBS and UC patients differ from HCs in terms of regional CT changes. 2) UC patients show CT changes in brain regions involved in somatosensory and viscerosensory processing and modulation. 3) IBS patients show CT changes in brain regions involved in the integration of affective, cognitive and interoceptive signals. 4) In UC patients, there are correlations between CT changes and duration of gut inflammation, reflecting the chronic influence of peripheral inflammation on the brain.
Materials and Methods
Subjects
A total 68 right-handed male and female subjects were recruited through the UCLA Digestive Disease Clinic and advertisements including HCs (n = 41; mean age = 28.2 years old, range = 19–48 years; 16 males), IBS with diarrhea (n = 11; mean age = 31.6 years old, range = 21–47 years; 2 males), and UC subjects (n = 16; mean age = 28. 6 years old, range 18–48 years; 10 males). 15 HCs and 5 IBS subjects of the respective samples have been included in a previously published gray matter volume analysis [9]. Exclusion criteria for all subjects comprised pregnancy, postpartum or nursing females, current substance abuse or dependence, abdominal surgery, any past or present neurological illness or trauma, claustrophobia or learning disability, and current psychiatric diagnosis. A diagnosis of IBS was made by a gastroenterologist or nurse practitioner with expertise in functional GI disorders based on the ROME II or ROME III symptom criteria during a clinical assessment [21], [22]. The diagnostic criteria include recurrent abdominal pain or discomfort associated with two or more of the following: 1) pain/discomfort is relieved/improved by defecation 2) the onset of pain/discomfort is related to a change in frequency of stool 3) the onset of pain/discomfort is related to a change in the form (appearance) of stool. In order to match the predominant bowel habit of UC patients, only IBS patients with diarrhea were used in this study. In addition, IBS subjects with current regular use of analgesic drugs (including narcotics, opioids and alpha2-delta ligands) were excluded. UC patients were diagnosed by a gastroenterologist, which was supported by biopsies obtained by endoscopy. During screening, all subjects completed the modified Mayo UCDAI (disease activity index for Stool frequency, rectal bleeding and physician rating of disease activity) to assess degree of current disease. A score of 0–1 is considered Remission, score of 2–4 mild disease, and >4 is considered active disease [23]. Exclusion criteria specific to the UC population were corticosteroid use within last 6 months, or use of any psychotropic medications. All procedures were approved by the UCLA Medical Institutional Review Board, and all subjects provided written informed consent. Questionnaires were completed before scanning to determine symptom type, severity, duration of symptoms, and abdominal sensation (UCLA Bowel Symptom Questionnaire, BSQ) [24], comorbid affective and mood disorders (Hospital Anxiety Depression Scale, HAD) [25], and IBS-related fears and anxiety (Visceral Sensitivity Index, VSI) [26], [27]. Details are shown in Table 1.
Table 1. Clinical and behavioral characteristics.
| HCs | IBS subjects | UC subjects | F | Sig. | |||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |||
| Sex (Male/Female) | 16/25 | 2/9 | 10/6 | ||||||||
| Age | 41 | 28.17 | 8.43 | 11 | 31.55 | 9.49 | 16 | 28.56 | 8.95 | .66 | .52 |
| Anxiety symptoms1 | 41 | 2.8 | 2.33 | 11 | 6.91 | 3.67 | 16 | 7.31 | 3.55 | 18.29 | <.01 |
| Depression symptoms1 | 41 | .85 | 1.22 | 11 | 2.64 | 2.73 | 16 | 3.44 | 3.54 | 7.77 | <.01 |
| Visceral Sensitivity Index 2 | 40 | 2.83 | 5.17 | 11 | 37.45 | 12.94 | 15 | 26.13 | 14.09 | 61.68 | <.01 |
| Overall Bowel Symptoms3 | 11 | 11.91 | 2.74 | 13 | 4.69 | 2.46 | 54.12 | <.01 | |||
| Abdominal Pain4 | 11 | 10.55 | 4.3 | 14 | 4.14 | 3.61 | 18.01 | <.01 | |||
| Abdominal Discomfort5 | 11 | 11.91 | 4.21 | 16 | 4.06 | 3.86 | 49.63 | <.01 | |||
| Duration of symptoms6 | 10 | 8.5 | 6.13 | 16 | 9.81 | 9.35 | 1.67 | .69 | |||
F = main effect of group from ANOVA and t-tests for four and two group comparisons, respectively.
1. HAD: Hospital Anxiety and Depression [25];
BSQ: Bowel Symptom Questionnaire [24].
3. BSQ Overall Symptoms in the Past week (0–20).
4. BSQ Abdominal Pain in the Past week (0–20).
5. BSQ Discomfort in the Past week (0–20).
6. BSQ Duration in years, derived from onset of symptom.
Statistically significant p<0.05.
Structural MRI Acquisition
All high-resolution T1-weighted brain images were collected at the UCLA Brain Mapping Center using a Siemens 3 Tesla Trio with magnetization prepared rapid gradient echo scanning parameters (TR = 2200 ms, TE = 3.26 ms, flip angle = 9°, duration = 9:03 mins, FOV = 256).
Data analysis
We employed the LONI pipeline for image preprocessing, cortical surface modeling and gray matter thickness analysis [19], [20]. Following a de-identification step, the structural neuroimaging data were converted from Digital Imaging and Communications in Medicine to ANALYZE 7.5 format, skull-stripped using the LONI Skull-Stripping Meta Algorithm pipeline workflow [28] and cortical surface models were generated using FreeSurfer 4.0 [29] (http://surfer.nmr.mgh.harvard.edu/fswiki and http://ucla.in/xSQPqT). Cortical grey matter thickness was computed at each point of the surface using the distance from the pial surface to the nearest point on the white matter surface. For numerical implementation, we first built the signed distance function [30], [31] of the white matter surface in 3D space and then computed the CT as the value on the signed distance function at those locations. The cortical surfaces and the corresponding CT maps were registered to the International Consortium for Brain Mapping (ICBM) brain surface [32] and then vertex-wise correspondences were established between all cortical surface models using a Conformal Metric Optimization method [33]. An experienced human brain researcher rated each brain surface reconstruction by visually inspecting the surfaces using LONI ShapeViewer (http://www.loni.ucla.edu/Software/ShapeViewer). The quality of surface reconstruction and accuracy of vertex labeling were assessed on the scale of 0 to 1 (0 = completely unacceptable; 1 = perfectly reconstructed and labeled). A threshold of 0.7 was selected as the criterion to reconstruct a subject's surface data to be included in the final analysis.
Region of interest analysis
We examined the CT change in several manually delineated regions of interest (ROIs) in each hemisphere based on previous studies [11], [14], [34]–[39]. These ROIs included insular subregions (anterior INS [aINS], mid INS [mINS], and posterior INS [pINS]), cingulate subregions (subgenual ACC [sgACC], pregenual ACC [pgACC], anterior midcingulate cortex [aMCC] and posterior MCC [pMCC]), orbitofrontal gyrus (OFG) (lateral OFG and medial OFG), pre- and postcentral gyri [32]. No subregions of pre- and postcentral gyri were drawn. The subregions of INS and cingulum were manually delineated on the 3D ICBM brain atlas [32] by two well-trained researchers with good command of neuroanatomical knowledge (Figure S1). The 3D ROI masks were transformed back onto the ICBM surface space by resampling the atlas map based on masks' Euclidean coordinates [33].
Statistical analysis
To determine potential protocol differences in ROIs, a general linear model (GLM) was applied to examine differences in total gray matter volumes within HCs as a function of protocol. Group differences in CT within ROIs as a function of group, sex and group*sex were determined using the GLM and weighted linear contrast analysis controlling for total gray matter volume, and age in SPSS v19. The contrasts testing the interaction between group and sex were weighted to eliminate any bias caused by unbalanced representation of sexes. Although this was a hypothesis driven study, we implemented a conservative procedure to adjust for multiple comparisons in order to control for type I error. Specifically, the false-discovery rate (FDR) for the 66 contrasts (11 bilateral ROIs [n = 22] for each of three independent contrasts) was held at 5% [40]–[42]. GLM and linear contrast analysis controlling for sex and age were also applied to examine differences in BSQ between IBS and UC subjects as well as group differences in non-BSQ clinical and behavioral characteristics including VSI and HAD. Significance was determined after controlling FDR at 5% [40], [41].
Correlation Analysis of Clinic Variables
Within group exploratory partial correlation analyses controlling for total gray matter volume, sex, and age were performed to characterize the association between subjects' clinic characteristics (BSQ, VSI, HAD and Mayo UCDAI) and regions showing significant group differences in CT. However, for the partial correlation between significant ROIs and symptom duration, we did not include age as a covariate, as these explanatory variables were significantly correlated, for IBS group (duration, r = .39, p = .036), and for UC group (duration, r = .67, p = .001) [43]. Significance was determined after controlling FDR at 5% [40], [41].
Results
Subject Characteristics
Subjects' clinical data are summarized in Table 1. Compared to the HCs, the two disease groups showed significantly higher measures of symptom related anxiety (VSI scores: IBS>HCs, F = 126.28, p<.001; UC>HCs, F = 71.97, p<.001; IBS>UC, F = 10.61, p = .002), anxiety symptoms (HAD scores: IBS>HCs, F = 17.03, p<.001; UC>HCs, F = 28.02, p<.001), and depression symptoms (HAD scores: IBS>HCs, F = 4.88, p = .031; UC>HCs, F = 15.16, p<.001). IBS subjects had significantly higher overall bowel symptoms scores, more abdominal discomfort and pain than UC subjects. Using the Mayo UCDAI [23], 5 of the UC subjects were in remission, 7 had mild disease and 4 had active disease (mild flare). There were no differences in terms of age of subjects and duration of GI symptoms.
Regional Cortical Thickness Changes Using ROI Analysis
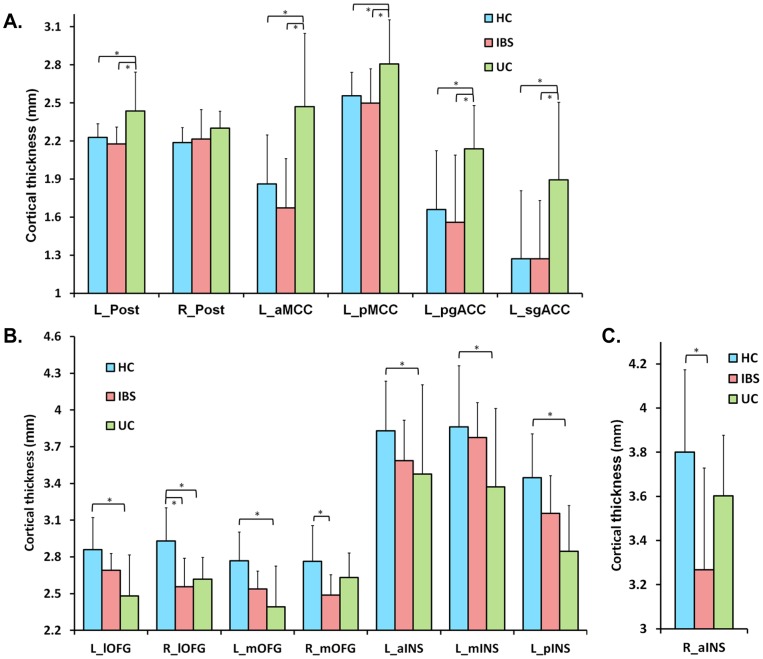
Regional CT differences were observed between UC, IBS and HCs. Mean CT values and statistical significance after FDR correction are shown in Table 2 and Table S1. As depicted in Figure 1A, compared to both IBS and HCs, UC subjects showed greater CT in left cingulate cortical subregions (aMCC, pMCC, pgACC and sgACC), and in left post central gyrus (statistically significant differences following FDR correction for multiple comparisons are shown in Figure 1A and marked with asterisks). As shown in Figure 1B, compared to both IBS and HCs, UC subjects had reduced CT in prefrontal regions (left medial and lateral OFG) and in left INS subregions (most significant in pINS). Following FDR correction for multiple comparisons, the results between HCs and UC remained significant even though some differences (OFG and INS) between IBS and UC groups were no longer significant (significant differences are shown in Figure 1B and marked with asterisks). All results remained significant after controlling for depression. However, after controlling for anxiety, the observed differences between HCs and UC subjects in left pMCC, aINS and mINS were no longer significant after FDR correction (Table 2). As shown in Figure 1C, compared to HCs, IBS subjects showed significantly reduced CT in right aINS after FDR correction, a difference that was not affected by controlling for anxiety and depression.
Table 2. Significant cortical thickness differences in the ROIs between HCs, IBS and UC subjects with and without controlling for anxiety and depression scores.
| ROI | Difference | F | P value | q value | F(A) | q(A) | F(D) | q(D) | |
| lOFG | L | HC>UC | 23.736 | <.00001 | .00023 | 15.898 | .00411 | 23.592 | .00020 |
| lOFG | R | HC>UC | 15.778 | .00019 | .00160 | 9.971 | .01654 | 14.000 | .00275 |
| HC>IBS | 17.591 | .00009 | .00086 | 12.647 | .00925 | 17.184 | .00102 | ||
| mOFG | L | HC>UC | 23.124 | .00001 | .00023 | 13.904 | .00715 | 26.905 | .00009 |
| mOFG | R | HC>IBS | 9.052 | .00383 | .01686 | 8.744 | .02156 | 9.802 | .01304 |
| Post | L | UC>HC | 9.895 | .00258 | .01216 | 9.397 | .01966 | 17.446 | .00102 |
| UC>IBS | 11.701 | .00113 | .00678 | 11.859 | .01001 | 15.595 | .00155 | ||
| aINS | L | HC>UC | 6.701 | .01207 | .04193 | 2.234 | .26245 | 7.698 | .02568 |
| aINS | R | HC>IBS | 18.098 | .00007 | .00082 | 12.384 | .00925 | 16.893 | .00102 |
| mINS | L | HC>UC | 10.304 | .00213 | .01173 | 3.859 | .14306 | 12.453 | .00489 |
| pINS | L | HC>UC | 30.701 | <.00001 | .00005 | 19.658 | .00150 | 29.416 | .00008 |
| aMCC | L | UC>HC | 18.231 | .00007 | .00008 | 11.277 | .01137 | 18.651 | .00080 |
| UC>IBS | 19.862 | .00004 | .00006 | 19.387 | .00150 | 21.006 | .00040 | ||
| pMCC | L | UC>HC | 8.870 | .00042 | .01724 | 2.278 | .26245 | 11.277 | .00758 |
| UC>IBS | 7.831 | .00690 | .02677 | 7.666 | .03185 | 9.082 | .01568 | ||
| pgACC | L | UC>HC | 11.713 | .00112 | .00678 | 8.839 | .02156 | 8.901 | .01607 |
| UC>IBS | 10.127 | .00232 | .01175 | 10.014 | .01654 | 9.690 | .01304 | ||
| sgACC | L | UC>HC | 11.754 | .00110 | .00678 | 8.692 | .02156 | 9.611 | .01304 |
| UC>IBS | 7.704 | .00734 | .02690 | 7.609 | .03185 | 7.559 | .02611 | ||
R: right; L: left; lOFG: lateral orbitofrontal gyrus; mOFG: medial orbitofrontal gyrus; Post: postcentral gyrus; aINS: aINSula; mINS: mid insula; pINS: posterior insula; aMCC: anterior mid cingulate cortex; pMCC: posterior mid cingulate cortex; pgACC: pregenual anterior cingulate cortex; sgACC: subgenual anterior cingulate cortex; q value: p value after FDR corrected at 5%, q value <.05 was considered significant; F(A): F score after controlling for anxiety; q(A): corrected p value after controlling for anxiety; F(D): F score after controlling for depression; q(D): corrected p value after controlling for depression.
Figure 1. Mean cortical thickness of the ROIs showing significant group differences.
(A) UC subjects showed the greatest CT in the regions of somatosensory and cingulate cortex. (B) In the subregions of OFG and INS, UCs had lower CT compared with HCs. (C) IBS subjects had lower CT in right aINS compared to HCs. Error bars reflect standard deviation. Asterisk indicates significant differences between groups (q<0.05) after controlling for sex, age, total gray matter volume and FDR correction.
Correlation of Cortical Thickness with Behavioral and Clinical Variables
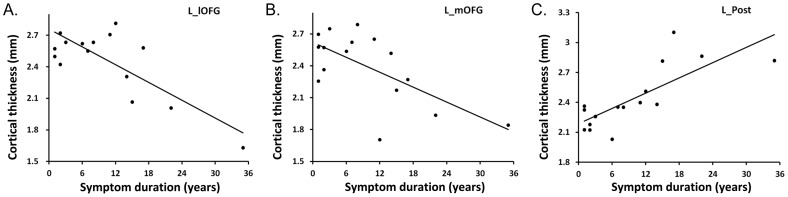
In the UC group, symptom duration was negatively correlated with CT in left lateral OFG (r = −.88, p = .0002, q = .0006, Figure 2A) and left medial OFG (r = −.78, p = .003, q = .003, Figure 2B) and was positively correlated with CT in left postcentral gyrus (r = .77, p = .003, q = .003, Figure 2C). Adding anxiety and depression scores as covariates did not alter the results. No significant correlations with other clinical parameters (including the Mayo UCDAI) were observed.
Figure 2. Correlation between cortical thickness and UC symptom duration.
(A) Cortical thickness in left lateral orbitofrontal gyrus (L_lOFG) and (B) left medial orbitofrontal gyrus (L_mOFG) were negatively correlated with symptom duration in UC group. (C) Cortical thickness in left postcentral gyrus (L_Post) showed large positive correlation with UC symptom duration.
Discussion
The primary goal of the current study was to assess regional CT differences between subjects with UC, and two comparison groups: a healthy control group and a disease control group without gut inflammation (IBS).The main findings of the study were: 1) Compared to both IBS and HCs, UC subjects showed greater CT in left cingulate cortical subregions, and in left primary somatosensory cortex (SI). 2) Compared with HCs, UC subjects showed lower CT in left OFG and in primary viscerosensory cortex (pINS). 3) Compared to HCs, IBS subjects showed lower CT in the interoceptive association cortex (aINS) in the right hemisphere. 4) There were large significant correlations of CT reductions in left OFG and CT increases in left SI with symptom duration in UC subjects, suggesting a role of chronic inflammation driven afferent input in these changes. The emerging pattern highlights significant differences in CT between patients with chronic gut inflammation, functional GI disorders and HCs, as well as some similarities.
Greater Regional CT in UC Patients
Somatosensory Cortex
In the current study, greater CT in primary somatosensory cortex (SI) was seen in the UC group. SI is part of the central pain processing network and its thickness is positively correlated with individual experimentally induced acute pain sensitivity in healthy subjects [44], [45]. Chronic pain in human patient populations has been shown to be associated with cortical reorganization and changes in SI activity [38], [46], [47]. For example, SI cortical thickening has been reported in patients with migraine [48] and temporomandibular pain [43]. It has been suggested that the critical factor for S1 to undergo structural reorganization may be the presence of constant sensory input to this brain region [38], [43], [47]. Additionally, a voxel based morphometry study showed increased left pre- and postcentral gyri in chronic back pain patients [39]. In the current study, CT of left postcentral gyrus in UC groups showed a large positive correlation with symptom duration, consistent with a possible etiologic role of chronically enhanced viscerosensory input to the brain due to sensitization of visceral afferent pathways by chronic mucosal inflammation. However, the degree of somatosensory cortex changes did not correspond to the subjective pain reports, as UC subjects had greater CT in somatosensory cortex, but reported lower abdominal pain and discomfort compared to patients with IBS. Even though the reason(s) for these apparent discrepancies between CT differences in SI and subjective pain reports are not known, one may speculate that the subjective experience of chronic clinical visceral pain (as opposed to acute experimentally induced pain) is more related to activity and related structural changes in interoceptive association cortex (e.g. the aINS), rather than to primary sensory cortex [4], [49].
Midcingulate Cortex
In the current study, compared to HCs and IBS subjects, the UC subjects had greater CT in subregions of the cingulate cortex, e.g. aMCC and pMCC. MCC is involved in emotion processing, skeletomotor regulation, chronic somatic and visceral pain, and along with the aINS (as part of the “salience network”) integrating information to form conceptual pain [50]–[54]. Several studies have reported abnormal MCC activation by acute noxious visceral stimulation in IBS subjects [55]–[58]. Supporting a possible effect of repeated nociceptive stimuli on MCC structure, repeated application of thermal pain stimuli to healthy subjects over a period of 8 days resulted in gray matter increases in both MCC and SI [59]. Together with the observed greater CT in SI, the findings in UC patients are most consistent with the presence of a constant sensory input from the gut, due to sensitization of visceral afferent pathways by chronic mucosal inflammation. This interpretation is also consistent with the fact that in the current study, the IBS group (e.g. without chronically recurring mucosal inflammation) did not show significant CT change compared to HCs. Further support for differential brain mechanisms underlying chronic visceral pain comes from a recent PET ligand study which showed differences in neurokinin-1 receptor (NK-1R) binding potential (e.g. receptor availability) between patients with IBD (including Crohn's disease and UC) and IBS subjects [60]. Compared to HCs, IBD patients had low NK-1R availability in ACC and MCC, while IBS showed this deficit to a lesser extent. Animal studies have shown that the substance P/NK-1R signaling system is involved in cytogenesis, has neurotrophic and neuroprotective functions and inhibits apoptosis [61]–[63]. This implies a differential involvement of such neuroplastic mechanisms in the two visceral pain syndromes. Our findings differ from those reported in two other chronic inflammatory conditions, e.g. Crohn's disease [14] and osteoarthritis [64]. In both of these studies lower gray matter in the MCC was observed compared to HCs. Differences in patient populations and analysis methodology make it difficult to directly compare these studies with the current report [14], [64].
Reduced Regional CT in IBS and UC Patients
INS Subregions
When compared to HCs, both disease groups showed lower CT in the INS, albeit in different subregions. Several studies in patients with chronic pain including IBS [7], [9], [12], [13], [64]–[68] compared to HCs, have found lower gray matter volumes and CT in the INS, even though subregions were often not specified. In the current study, UC compared to HCs had significantly reduced CT in the left pINS (observed differences in mINS were no longer seen after controlling for anxiety). It is likely that chronically enhanced afferent input from the gut due to recurrent mucosal inflammation is primarily associated with CT changes in the pINS, which represents the primary interoceptive cortex [37], [69]. Even though there was no significant correlation between symptom duration or other behavioral measures with CT changes in the pINS, a chronic low back pain study showed that recovery of CT in pINS (and secondary somatosensory cortex) was correlated with reduction of pain intensity after treatment [12], implicating chronic nociceptive and inflammation related signaling as a factor in these CT changes.
In contrast to the UC group, IBS subjects had lower CT in a different subregion of the INS, e.g. the right aINS compared to HCs. The aINS functions as interoceptive association cortex integrates interoceptive input with emotional, salient and cognitive inputs, and provides output to autonomic and pain modulation systems [37], [53], [69]–[71]. The aINS also plays a central role in prediction, error processing, and self awareness of sensations [37], [71]. Even though both patient groups had greater affective scores compared to HCs, IBS subjects reported more abdominal pain and discomfort compared to the UC group. However, there was no significant correlation of the observed changes with affective measures, symptom scores or duration of symptoms in IBS subjects.
Orbitofrontal Gyrus
UC subjects compared to HCs showed lower CT in the bilateral OFG, a brain region which plays an important role in interoception, emotion evaluation and regulation, and in cognitive reappraisal [43], [72], [73]. Decreased gray matter in OFG has also been found in other chronic pain disorders with inflammatory/nociceptive drive including hip osteoarthritis [64], low back pain [39] and migraine [74]. A negative correlation between left OFG thickness and symptom duration was observed in UC subjects, suggesting a role of chronic nociceptive input in the observed CT reductions. In contrast, no such correlation between IBS symptom duration and OFG structure were observed.
Limitations
Limitations of the study include the small sample size of the IBS and UC population, the group differences in level of anxiety and depression symptoms, and the heterogeneity of the groups in terms of sex. However, controlling for anxiety and depression, most of the observed results remained significant. In addition, our GLM and linear contrast were weighted to eliminate any bias caused by umbalanced representation of sexes. Furthermore, the fact that large correlations of some structural changes with disease duration were observed in the UC subjects, and the fact that some of the findings were similar to reports in subjects with other chronic inflammatory conditions [13], [64], makes it unlikely that the findings are confounded by these limitations. Future, longitudinal studies in larger patient populations, including the correlation of plasma and mucosal inflammatory disease markers with structural brain changes both during disease flares and remissions are needed in order to better understand the role of colonic inflammation in remodeling of the brain. In such larger studies, presence of comorbidities in the IBS group, as well as differences in the impact of the IBS and UC on daily life activities and social interactions should be taken into account.
Conclusions
To our knowledge, this study represents the first comparison of brain structure between UC patients and both HCs and IBS subjects. The findings demonstrate significant differences in CT between UC and HC subjects, and differences between the two disease groups. Based on the correlation of structural changes with symptom duration in IBD, one may speculate that the observed gray matter reorganization of IBD subjects represents a consequence of chronic viscerosensory input to the brain due to sensitization of visceral afferent pathways by recurrent gut inflammation. The mechanisms by which such increased viscerosensory to the brain input can produce both increases and decreases of grey matter in different brain regions remains to be determined.
Supporting Information
Manually delineated subregions of interest on the 3D International Consortium for Brain Mapping brain atlas. (A) Subregions of cingulate cortex: anterior mid cingulate cortex (aMCC), posterior mid cingulate cortex (pMCC), pregenual anterior cingulate cortex (pgACC) and subgenual anterior cingulate cortex (sgACC). (B) Subregions of insula: aINSula (aINS), mid insula (mINS) and posterior insula (pINS).
(TIF)
Mean cortical thickness.
(DOCX)
Acknowledgments
The authors would like to thank Ahmanson Lovelace Brain Mapping Center for expert technical assistance.
Funding Statement
This work was funded in part by the National Institutes of Health through Center for Neurobiology (CNS) grants: K08 DK071626, R03 DK084169, K23DK073451, R01 AT007137, P50 DK064539, R01 DK048351, and LONI grants: NIBIB 9P41EB015922-15, NCRR 2-P41-RR-013642-15, NCRR U54 RR021813, U24-RR025736, U24-RR021992. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bielefeldt K, Davis B, Binion DG (2009) Pain and inflammatory bowel disease. Inflamm Bowel Dis 15: 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, et al. (2008) Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci 28: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, et al. (2000) Perceptual responses in patients with inflammatory and functional bowel disease. Gut 47: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, et al. (2005) Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115: 398–409. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, et al. (2007) Striatal grey matter increase in patients suffering from fibromyalgia – a voxel-based morphometry study. Pain 132 Suppl 1S109–116. [DOI] [PubMed] [Google Scholar]
- 6.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, et al.. (2010) Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139: 48–57 e42. [DOI] [PMC free article] [PubMed]
- 7. Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, et al. (2008) Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology 70: 153–154. [DOI] [PubMed] [Google Scholar]
- 8. Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, et al. (2009) No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain 143: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labus J, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, et al.. (2013) Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. [DOI] [PMC free article] [PubMed]
- 10. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, et al. (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24: 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, et al. (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125: 89–97. [DOI] [PubMed] [Google Scholar]
- 12. Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, et al. (2011) Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 31: 7540–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frokjaer JB, Bouwense SA, Olesen SS, Lundager FH, Eskildsen SF, et al.. (2012) Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin Gastroenterol Hepatol 10: 434–438 e431. [DOI] [PubMed]
- 14.Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, et al.. (2013) New insights into the brain involvement in patients with Crohn's disease: a voxel-based morphometry study. Neurogastroenterol Motil. [DOI] [PubMed]
- 15. Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, et al. (2011) Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol 186: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, et al. (2012) Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum 64: 371–379. [DOI] [PubMed] [Google Scholar]
- 17. Jones AK, Huneke NT, Lloyd DM, Brown CA, Watson A (2012) Role of functional brain imaging in understanding rheumatic pain. Curr Rheumatol Rep 14: 557–567. [DOI] [PubMed] [Google Scholar]
- 18. Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I (2010) Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum 62: 2930–2940. [DOI] [PubMed] [Google Scholar]
- 19. Dinov ID, Torri F, Macciardi F, Petrosyan P, Liu Z, et al. (2011) Applications of the pipeline environment for visual informatics and genomics computations. BMC Bioinformatics 12: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinov I, Lozev K, Petrosyan P, Liu Z, Eggert P, et al.. (2010) Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS One 5. [DOI] [PMC free article] [PubMed]
- 21.Drossman DA (2000) Rome II: the functional gastrointestinal disorders: diagnosis, pathophysiology, and treatment: a multinational consensus. McLeanVA: Degnon Associates. xxvii, 764 p. [Google Scholar]
- 22. Drossman DA (2006) The functional gastrointestinal disorders and the Rome III process. Gastroenterology 130: 1377–1390. [DOI] [PubMed] [Google Scholar]
- 23. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, et al. (2008) Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 14: 1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA (2001) Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol 96: 3341–3347. [DOI] [PubMed] [Google Scholar]
- 25. Mykletun A, Stordal E, Dahl AA (2001) Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry 179: 540–544. [DOI] [PubMed] [Google Scholar]
- 26. Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, et al. (2004) The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther 20: 89–97. [DOI] [PubMed] [Google Scholar]
- 27. Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD (2007) The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med 69: 89–98. [DOI] [PubMed] [Google Scholar]
- 28.Leung KTK (2011) Principal Ranking Meta-Algorithms: UNIVERSITY OF CALIFORNIA, LOS ANGELES.
- 29. Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulder W, Osher S, Sethian JA (1992) Computing interface motion in compressible gas dynamics. Journal of Computational Physics 100: 209–228. [Google Scholar]
- 31. Osher S, Sethian JA (1988) Fronts propagating with curvature-dependent speed: algorithms based on Hamilton-Jacobi formulations. Journal of Computational Physics 79: 12–49. [Google Scholar]
- 32. Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, et al. (2001) A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356: 1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Y, Lai R, Gill R, Pelletier D, Mohr D, et al. (2011) Conformal metric optimization on surface (CMOS) for deformation and mapping in Laplace-Beltrami embedding space. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention 14: 327–334. [DOI] [PubMed] [Google Scholar]
- 34. Tillisch K, Mayer EA, Labus JS (2011) Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer EA, Bushnell MC, International Association for the Study of Pain. (2009) Functional pain syndromes: presentation and pathophysiology. Seattle: IASP Press. xviii, 580 p. [Google Scholar]
- 36.Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, et al.. (2012) New insights into the brain involvement in patients with Crohn's disease: a voxel-based morphometry study. Neurogastroenterol Motil. [DOI] [PubMed]
- 37. Craig AD (2009) How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- 38. May A (2008) Chronic pain may change the structure of the brain. Pain 137: 7–15. [DOI] [PubMed] [Google Scholar]
- 39.Ung H, Brown JE, Johnson KA, Younger J, Hush J, et al.. (2012) Multivariate Classification of Structural MRI Data Detects Chronic Low Back Pain. Cereb Cortex. [DOI] [PMC free article] [PubMed]
- 40. Benjamini Y, Krieger AM, Yekutieli D (2006) Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507. [Google Scholar]
- 41. Benjamini Y, Hochberg Y (2000) On the Adaptive Control of the False Discovery Rate in Multiple Testing with Independent Statistics. Journal of Educational and Behavioral Statistics 25: 60–83. [Google Scholar]
- 42. Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution. Methods in Ecology and Evolution 2: 278–282. [Google Scholar]
- 43. Moayedi M, Weissman-Fogel I, Crawley AP, Goldberg MB, Freeman BV, et al. (2011) Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage 55: 277–286. [DOI] [PubMed] [Google Scholar]
- 44. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 45. Erpelding N, Moayedi M, Davis KD (2012) Cortical thickness correlates of pain and temperature sensitivity. Pain 153: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 46. Moseley GL, Flor H (2012) Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair 26: 646–652. [DOI] [PubMed] [Google Scholar]
- 47. Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, et al. (2012) Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci 32: 14874–14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DaSilva AF, Granziera C, Snyder J, Hadjikhani N (2007) Thickening in the somatosensory cortex of patients with migraine. Neurology 69: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blankstein U, Chen J, Diamant NE, Davis KD (2010) Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 138: 1783–1789. [DOI] [PubMed] [Google Scholar]
- 50. Taylor KS, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vogt BA, Berger GR, Derbyshire SW (2003) Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 18: 3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, et al. (2010) AINSula integrates information about salience into perceptual decisions about pain. J Neurosci 30: 16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meier ML, Brugger M, Ettlin DA, Luechinger R, Barlow A, et al. (2012) Brain activation induced by dentine hypersensitivity pain – an fMRI study. J Clin Periodontol 39: 441–447. [DOI] [PubMed] [Google Scholar]
- 55. Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, et al. (2009) Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage 47: 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, et al. (2002) Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol 97: 319–327. [DOI] [PubMed] [Google Scholar]
- 57. Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, et al. (2003) Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 103: 99–110. [DOI] [PubMed] [Google Scholar]
- 58. Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, et al. (2005) Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 65: 1268–1277. [DOI] [PubMed] [Google Scholar]
- 59. Teutsch S, Herken W, Bingel U, Schoell E, May A (2008) Changes in brain gray matter due to repetitive painful stimulation. Neuroimage 42: 845–849. [DOI] [PubMed] [Google Scholar]
- 60.Jarcho JM, Feier NA, Bert A, Labus JA, Lee M, et al.. (2013) Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain. Pain. [DOI] [PMC free article] [PubMed]
- 61. Lallemend F, Lefebvre PP, Hans G, Rigo JM, Van de Water TR, et al. (2003) Substance P protects spiral ganglion neurons from apoptosis via PKC-Ca2+-MAPK/ERK pathways. J Neurochem 87: 508–521. [DOI] [PubMed] [Google Scholar]
- 62. Tulloch I, Ghazaryan N, Mexhitaj I, Ordonez D, Angulo JA (2011) Role of neurokinin-1 and dopamine receptors on the striatal methamphetamine-induced proliferation of new cells in mice. Brain Res 1399: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Angulo JA (2011) Synergism between methamphetamine and the neuropeptide substance P on the production of nitric oxide in the striatum of mice. Brain Res 1369: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A (2009) Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 29: 13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Valfre W, Rainero I, Bergui M, Pinessi L (2008) Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 48: 109–117. [DOI] [PubMed] [Google Scholar]
- 66. Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, et al. (2007) Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 27: 4004–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, et al. (2005) Gray matter decrease in patients with chronic tension type headache. Neurology 65: 1483–1486. [DOI] [PubMed] [Google Scholar]
- 68. Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, et al. (2013) Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One 8: e73932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farb NA, Segal ZV, Anderson AK (2012) Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex 23: 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis M, Haviland-Jones JM, Barrett LF (2008) Handbook of emotions. New York: Guilford Press. xvi, 848 p., [844] p. of plates p.
- 71. Paulus MP, Stein MB (2006) An insular view of anxiety. Biol Psychiatry 60: 383–387. [DOI] [PubMed] [Google Scholar]
- 72. Rolls ET, Grabenhorst F, Parris BA (2008) Warm pleasant feelings in the brain. Neuroimage 41: 1504–1513. [DOI] [PubMed] [Google Scholar]
- 73. Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, et al. (2005) Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci 5: 156–168. [DOI] [PubMed] [Google Scholar]
- 74. Kim JH, Suh SI, Seol HY, Oh K, Seo WK, et al. (2008) Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28: 598–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Manually delineated subregions of interest on the 3D International Consortium for Brain Mapping brain atlas. (A) Subregions of cingulate cortex: anterior mid cingulate cortex (aMCC), posterior mid cingulate cortex (pMCC), pregenual anterior cingulate cortex (pgACC) and subgenual anterior cingulate cortex (sgACC). (B) Subregions of insula: aINSula (aINS), mid insula (mINS) and posterior insula (pINS).
(TIF)
Mean cortical thickness.
(DOCX)