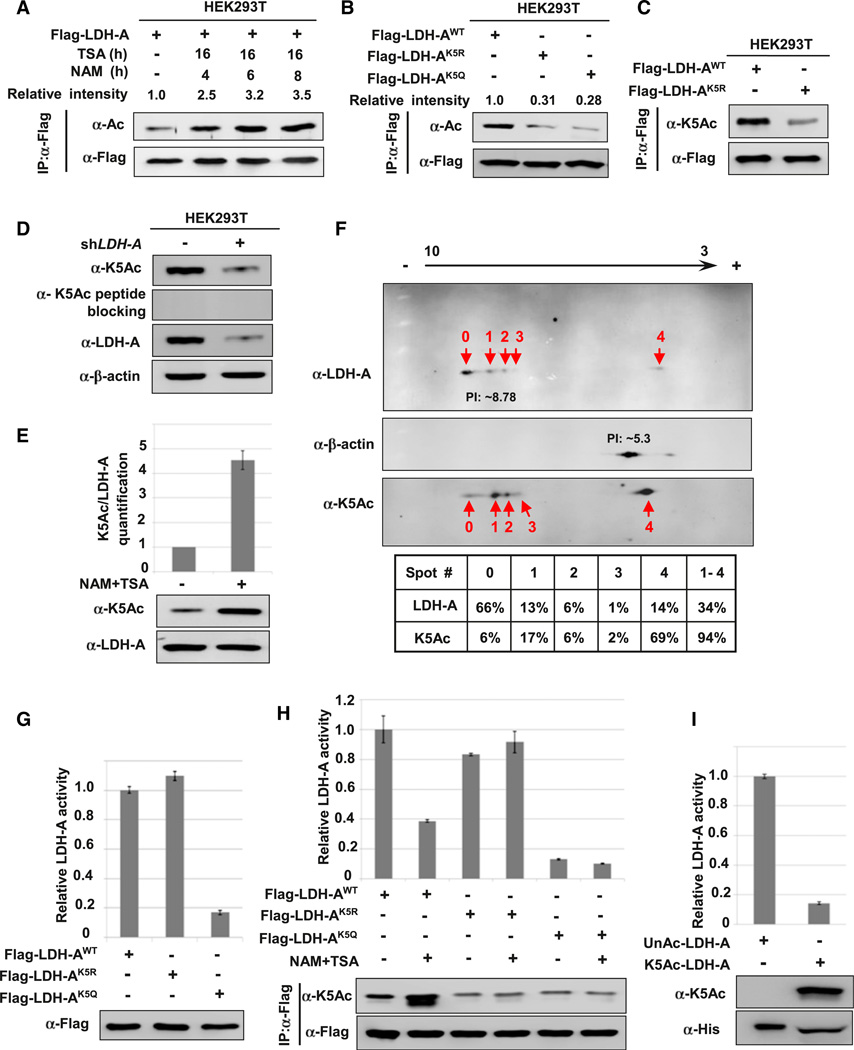
Figure 1. Acetylation at Lys-5 Decreases LDH-A Enzyme Activity.
(A) LDH-A is acetylated. Flag-LDH-A was transfected into 293T cells followed by treatment with deacetylase inhibitors TSA and NAM for indicated time. LDH-A acetylation and protein levels were analyzed by western blot with indicated antibody. Relative ratios of acetylation were calculated from normalizing against Flag-LDH-A.
(B) Mutation of K5 decreases LDH-A acetylation. The indicated plasmids were transfected into 293T cells and proteins were immunoprecipitated for western blotting.
(C) Characterization of acetyl-LDH-A(K5) antibody. The indicated plasmids were transfected into 293T cells, acetylation level of immunoprecipitated Flag-LDH-A was measured by direct western bloting using the acetyl-LDH-A (K5) antibody (α-K5Ac).
(D) Endogenous LDH-A is acetylated on lysine 5. Cell lysate from scramble or LDH-A shRNA knockdown stable cells were probed with indicated antibodies.
(E) Treatment with NAM and TSA increases endogenous LDH-A acetylation; 293T cells were treated with TSA and NAM. Endogenous LDH-A protein levels and acetylation of K5 were determined by western blotting with indicated antibodies (bottom panel). Relative K5-acetylated LDH-A over total LDH-A protein was quantified (top panel). Error bars represent ± SD for triplicate experiments.
(F) Quantitative analysis of endogenous LDH-A acetylation at K5 by isoelectric focusing (IEF) analysis; 293T cells lysate were separated by IEF, followed by western blotting using indicated antibodies. Relative LDH-A K5 acetylation and LDH-A protein levels for each spot were quantified by intensity, and the relative percentage of each spot is calculated and listed below the western blot panels.
(G) K5Q mutant decreases LDH-A enzyme activity. Flag-tagged wild-type and mutant LDH-A protein were expressed in 293T cells and purified by immuno-precipitation. The enzyme activity was measured and normalized against protein level. Relative enzyme activities of triplicate experiments with ± SD are presented.
(H) NAM and TSA treatment decreases the enzyme activity of wild-type, but not mutant LDH-A. Flag-tagged wild-type and mutant LDH-A protein were expressed in 293T cells and treated with or without NAM and TSA, then purified by immunoprecipitation. The LDH-A enzyme activity was measured and normalized against protein level. Relative enzyme activities of triplicate experiments ± SD are presented.
(I) Acetylated LDH-A has lower enzyme activity. Recombinant un-acetylated and K5-acetylated LDH-A protein were prepared by the system of genetically encoding Nε-acetyllysine in E. coli. The enzyme activity was measured and normalized against protein level. Relative enzyme activities of triplicate experiments ± SD are presented. See also Figure S1.