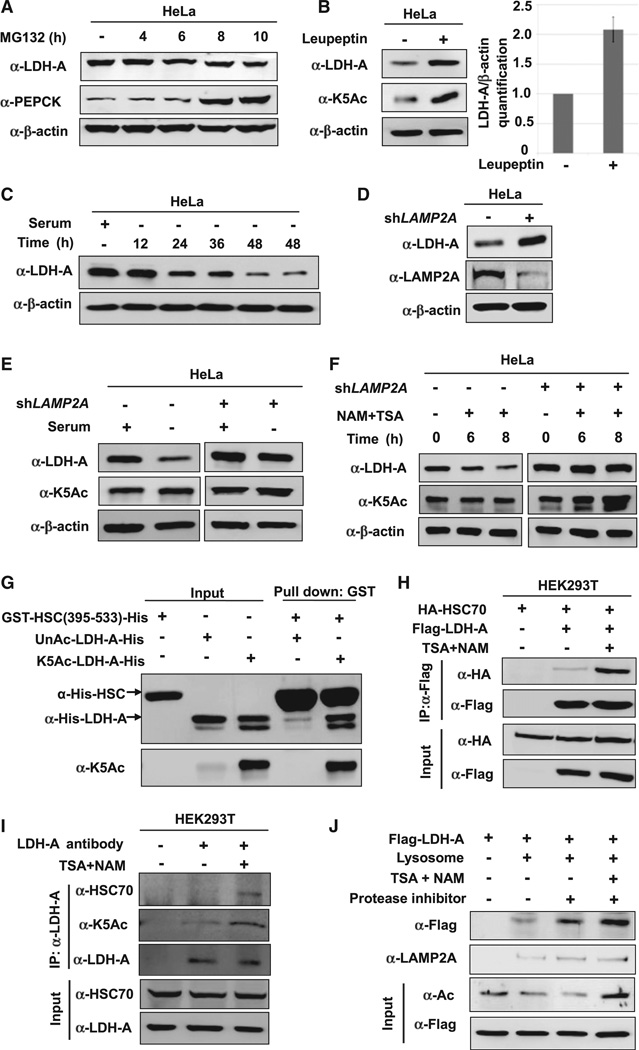
Figure 4. Acetylation Promotes LDH-A Degradation via CMA.
(A) LDH-A is not degraded by the ubiquitin-proteasome system (UPS). HeLa cells were treated with a proteasome inhibitor MG132 and the LDH-A protein level was analyzed by western blotting. PEPCK, a known substrate of UPS, was included as a control.
(B) Leupeptin accumulates K5-acetylated and total LDH-A protein. HeLa cells were either untreated or treated with leupeptin for 48 hr. The levels of total and acetylated LDH-A were determined by western blotting. LDH-A level was normalized against β-actin. Error bars represent ± SD of triplicated experiments.
(C) Serum withdrawal decreases LDH-A protein. LDH-A level was determined by western blotting after serum withdrawal for different lengths of time, as indicated in HeLa cells.
(D) LAMP2A knockdown accumulates LDH-A. LAMP2A was stably knocked down in HeLa cells by shRNA. The knockdown efficiency and LDH-A protein level were determined by western blotting. (E and F) LAMP2A knockdown blocks the effect of serum deprivation or NAM and TSA treatment on LDH-A protein levels. HeLa cell pools stably expressing LAMP2A shRNA were cultured with or without serum (E) or NAM and TSA (F). The levels of K5-acetylated and total LDH-A protein were determined by western blotting.
(G) Acetylation at K5 increases LDH-A binding to the HSC70 C-terminal domain in vitro. Recombinant unacetylated and K5-acetylated LDH-A protein were prepared by the system of genetically encoding Nε-acetyllysine in E. coli. GST-HCS70 C-terminal domain (from 395 to 533 amino acids) was used in an in vitro binding assay to pulldown the purified LDH-A.
(H and I) Inhibition of deacetylases increases overexpressed or endogenous LDH-A-HSC70 binding. Indicated plasmids were co-transfected into 293T cells, followed by NAM and TSA treatment. LDH-A-HSC70 binding was determined by immunoprecipitation-western analysis (H). The 293T cells were untreated or treated with NAM and TSA (I). Endogenous LDH-A-HSC70 binding was determined by immunoprecipitation and western blot analysis.
(J) Inhibition of deacetylases promotes lysosomal uptake of LDH-A. Flag-tagged LDH-A was immunopurified from 293T cells untreated or treated with deacetylase inhibitors TSA and NAM. The immunoprecipitated LDH-A was incubated with the lysosomes isolated from rat liver. Lysosomes were re-isolated and the associated LDH-A (either inside or binding to the surface) were determined by western blotting.
See also Figure S4.