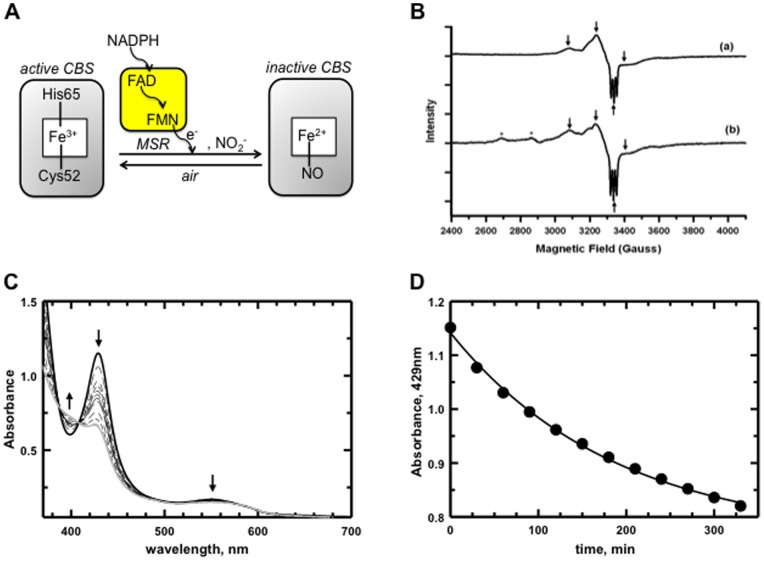
Figure 2. Model for and spectroscopic evidence of formation of FeII-NO CBS in the presence of MSR/NADPH.
(A) FeIII-CBS catalyzes the condensation of cysteine (Cys) and homocysteine (Hcy) to give H2S and cystathionine (Cyst). The latter is subsequently cleaved to give cysteine, which is utilized for glutathione (GSH) synthesis. In the presence of NADPH/MSR and nitrite, FeII-NO CBS is formed, rendering CBS inactive. (B) EPR spectra of FeII-NO CBS, obtained with FeIII-CBS (65 µM), treated with dithionite (6 mM) (upper) or NADPH (2 mM)/MSR (20 µM) (lower) and sodium nitrite (10 mM) in 0.1 M HEPES buffer, pH 7.4 at 37°C. The spectra were recorded using the conditions described previously [26]. The arrows indicate g values of 2.17, 2.076, 2.008 and 1.97, respectively. The presence of additional EPR signals in the spectrum of NADPH/MSR-dependent CBS-catalyzed nitrite reduction can be attributed to the incomplete reduction of paramagnetic FeIII-CBS. (C) UV-visible spectra were recorded every 10 min under anaerobic conditions for the reaction between FeII-CBS (generated by reduction of FeIII-CBS (10 µM) with MSR (2 µM)/NADPH (1 mM)) and nitrite (10 mM) in 0.1 M HEPES buffer, pH 7.4, at 37°C. (B) Time-dependent conversion of FeIII-CBS (429 nm) to FeII-NO-CBS (394 nm).