Figure 5. Depiction of effector functions during the E. chaffeensis life cycle in a mammalian monocyte.
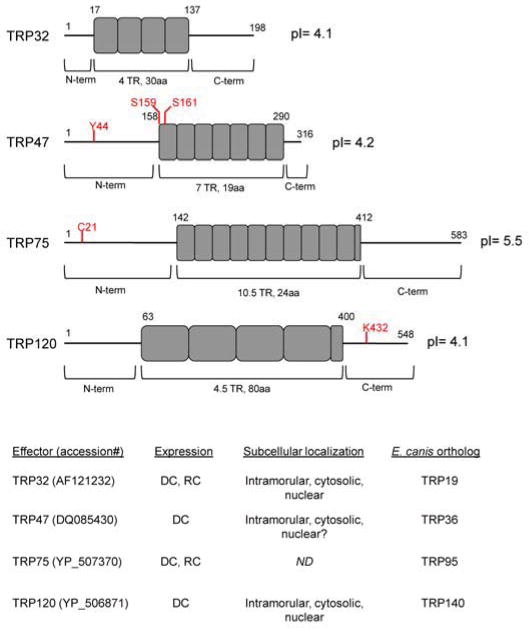
1, DC bacteria bind DNaseX, a GPI-anchored receptor as well as other GPI-anchored proteins within caveoli at the surface of the monocyte. ECH_1038 and TRP120 mediate this interaction and facilitate bacterial endocytosis. 2, DC bacteria establish an intracellular niche in a membrane-bound vacuole, to which early-endosomal proteins, including Rab5a and VAMP2, are recruited. This inclusion does not undergo phagolysosomal fusion. Ehrlichial effectors, including TRP32, TRP47, TRP120, and Ank200 are secreted into the intramorular space via T1SS and translocate across the vacuolar membrane (via an undefined mechanism) into the host cytosol. In the cytosol these effectors are substrates of host post translational modification machinery (TRP47 is phosphorylated; TRP120 is SUMO and Ub modified), and interact with diverse host proteins to alter cell processes. Through an NLS-independent mechanism, ehrlichial effectors are also translocated to the host nucleus where Ank200 and TRP120 bind host genomic DNA at Alu-Sx elements and GC motifs, respectively, and modulate host gene expression. Changes in gene expression include upregulation of signaling genes, including NFκB, and STAT1, as well as downregulation of innate immune-related genes JAK2, TLR2, and TLR4. 3, 1 h post infection, DC bacteria transition into the RC morphology and replicate by binary fission every 8 h for the following 48 h. During this period ehrlichial effectors continue to modulate host cell processes. Host cell signaling is disrupted, including IFNγ-mediated JAK/STAT signaling, as a means of avoiding host innate immune responses. Vesicular trafficking is exploited to obtain cholesterol and iron (via transferrin and transferrin receptors) necessary for replication. Mitochondria redistribute to the periphery of the ehrlichial inclusion. T4SS effector, ECH_0825, translocates to these mitochondria to inhibit Bax-induced apoptosis and ROS production, thereby permitting intracellular survival. 4, 72 h post infection RC morphology transitions into DC cell type. Effectors, including T1SS TRP47 and TRP120 translocate across the vacuolar membrane and interact with host cytoskeletal proteins, including β-actin, γ-actin, γ-tubulin, and myosin X, as well as accessory proteins, including components of the ARP2/3 complex and CAP1, to facilitate exocytosis or filopodium formation (for direct cell-cell transfer). Late in infection ehrlichial effectors induce host apoptosis, possibly to facilitate bacterial release.