Figure 8.
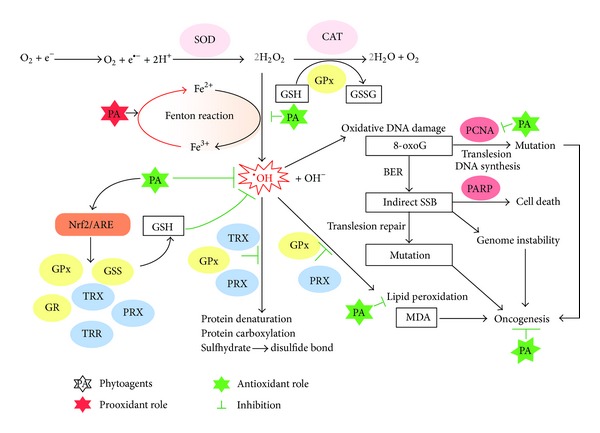
Summary of mechanisms of action of phytoagents in chemoprevention and chemotherapeutics through modulating oxidative stress. In the presence of ferrous ions (or other transition metal ions), phytoagents recycle the metal ion and thus promote the Fenton reaction that generates the highly reactive hydroxyl radical from hydrogen peroxide. Such prooxidant effects of phytoagents in the presence of metal ion can overwrite their cytoprotective roles because the production of ROS may be faster than the induction of antioxidant defense. Hydrogen peroxide imposes oxidative damage on biomolecules, such as proteins, lipids, and DNA, and leads to protein carbonylation, lipid peroxidation, and DNA base oxidation, which can be prevented by phytoantioxidants. Phytoantioxidants can activate Nrf2/ARE signaling and thus transcriptionally upregulate a panel of antioxidant genes that can provide further antioxidant capacity. Glutathione synthetase (GSS) can raise the level of glutathione (GSH) which can reduce oxidative damage by scavenging hydroxyl radicals, which otherwise cause oxidative DNA damage and increase the chance of point mutation and genome instability during the DNA repair process while glutathione reductase (GR) recycles the oxidized form of GSH and maintains the level of the reduced form of GSH. Glutathione peroxidase (GPx), thioredoxin (TRX), and peroxiredoxin (PRX) can prevent oxidative insults on proteins and lipids.
