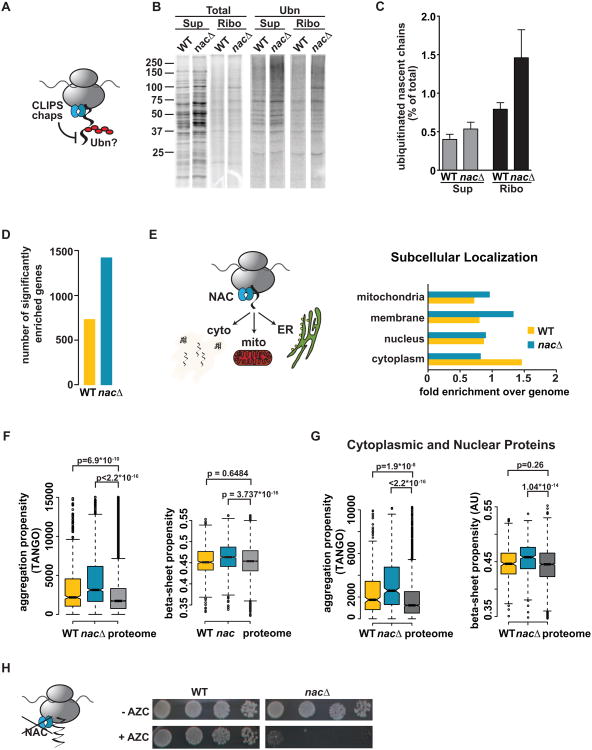
Figure 6. The ribosome-associated chaperone NAC protects nascent chains from cotranslational ubiquitination.
(A) Hypothesis: Ribosome-bound chaperones such as NAC protect nascent chains from ubiquitination (B) Pulse-labeling followed by polyUb-affinity isolation in egd1/2Δbtt1Δ (nacΔ) shows increased ubiquitination compared to WT. (C) Quantification: Mean ± SEM (n=4). (D) Deletion of NAC increases in the number of mRNAs encoding cotranslationally ubiquitinated nascent chains. (E) Deletion of NAC enhances ubiquitination of nascent chains trafficked to the secretory pathway and mitochondria. (Left) Role of NAC in targeting to the ER and mitochondria. (Right) Subcellular localization of ub+ proteins in WT and nacΔ datasets. (F) Deletion of NAC causes ubiquitination of nascent chains with very high aggregation- and beta-sheet propensity compared to the proteome. (G) As in (F) but analysis was restricted to cytoplasmic and nuclear proteins. (H) Synthetic effect of loss of NAC with impaired cotranslational folding: nacΔ cells are hypersensitive to AZC. Plates were grown for 3 days at 30 °C. See also Figure S5.