Abstract
Tremendous progress has been made towards the goal of global elimination of lymphatic filariasis (LF) transmission by 2020. The number of endemic countries reducing LF transmission through mass drug administration continues to increase, and therefore, the need for effective post-intervention surveillance also continues to increase. Togo is the first sub-Saharan African country to implement LF surveillance, and it has 6 years of experience with this passive surveillance system. We herein report the results of a recent evaluation of the Togolese LF surveillance system, including an evaluation of blood donors as a surveillance population, and provide updated results of ongoing surveillance, including expansion in remote areas. Since implementation of LF surveillance in 2006, only three cases of positive Wuchereria bancrofti filaremia have been detected, suggesting that interruption of transmission has been sustained. Given the impracticality of validating the surveillance system in the absence of ongoing transmission, we confirmed the lack of transmission through a nationwide reassessment survey.
Background
Lymphatic filariasis (LF) is a disfiguring and disabling disease caused by the filarial worms Wuchereria bancrofti, Brugia malayi, and B. timori. It is endemic in 72 countries worldwide and a leading cause of chronic disability.1,2 In response to the World Health Assembly's resolution to eliminate LF as a public health problem by 2020,3 the Global Program to Eliminate LF (GPELF) was organized in 2000, with the dual goal of worldwide elimination of LF transmission and alleviation of morbidity among those individuals with LF-induced hydrocele or lymphedema.
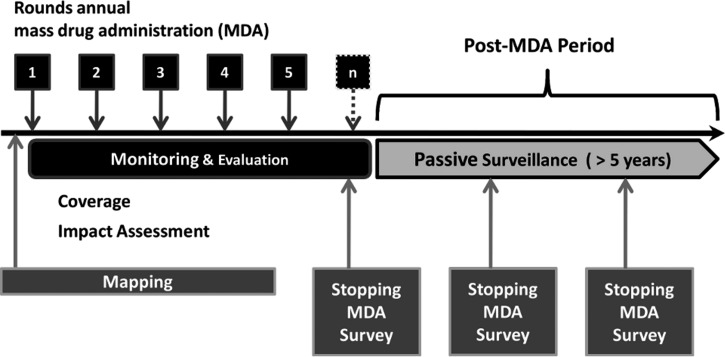
The World Health Organization (WHO) has provided guidelines outlining specific steps on the road to elimination of LF transmission (Figure 1). Mapping identifies LF-endemic areas of the country and is followed by at least five rounds of annual mass drug administration (MDA) in endemic areas. Concurrent monitoring and evaluation (M&E) are conducted in the areas under MDA to ensure that targets are met. A transmission assessment survey (TAS) determines when the MDA can be stopped. On cessation of MDA, the country enters a surveillance period of at least 5 years.4,5 Surveillance can take the form of an ongoing passive surveillance system, or it can be executed by repeating the TAS at 3 and 5 years.5 If passive surveillance is used, WHO guidelines state that it should be implemented as early as possible, because approximately 5 years of post-MDA surveillance data are required to confirm the sustained absence of transmission.4,5 In contrast to the specific guidelines for mapping, MDA, and M&E, WHO offers little guidance regarding passive surveillance. Four potential target populations are suggested (military recruits, university students, blood donors, and hospitalized patients), but the details of implementation are left to individual programs.4,5
Figure 1.
Schematic representation of WHO guidelines for LF elimination activities in Togo from 2010 to 2011.
Togo was one of the first African nations to implement a national LF elimination program. Seven of the country's thirty-five medical districts were LF-endemic before onset of MDA in 2000; the last MDA in Togo was conducted in 2009.6 A nationwide laboratory-based LF surveillance system, developed in cooperation with the US Centers for Disease Control and Prevention (CDC), covering all 35 districts and designed to be sustainable in resource-poor settings was implemented in 2006 and has been described in detail in the work by Mathieu and others.7 The technicians in this laboratory-based surveillance system review nocturnal malarial thick blood smears collected from patients at district hospitals (or equivalent inpatient facilities) throughout the country for the presence of microfilariae. During the first 2 years of surveillance, the system detected two cases of positive microfilaremia among 8,050 persons whose nocturnal thick blood smears were reported to the surveillance system.7 At the request of the National Program to Eliminate LF in Togo (NPELF), the CDC provided an evaluation of the first 2 years of the surveillance system. This paper describes the evaluation, which included assessment of possible alternative ways to implement LF surveillance in Togo, and reports on the implementation of suggested improvements.
Methods
Evaluation of the LF surveillance system.
The evaluation was based on criteria outlined in CDC guidelines for surveillance system evaluations.8,9 Specific objectives included (1) creation of a map plotting the geographic catchment area of the system during the first 2 years, (2) development of an improved protocol for surveillance, (3) nationwide reassessment of LF transmission status, and (4) assessment of other possible LF surveillance systems.
Mapping the geographic catchment area.
The geographic catchment area of the laboratory-based surveillance system was charted by plotting the village of residence for each patient tested in 2006 and 2007.7 Coordinates for each village were obtained from a Ministry of Health (MOH) global positioning system (GPS) database and prior neglected tropical diseases (NTD) mapping studies (unpublished data). In addition, the MOH located villages not found in these databases by visiting the villages and collecting coordinates using handheld GPS units. The GPS coordinates for each village of residence were then plotted on a map of Togo using ArcView GIS software (ESRI, Redlands, CA).
Improved surveillance protocol.
The laboratory-based LF surveillance system in Togo has previously been described7 and relies on detection of circulating microfilariae in thick blood smears drawn between 10:00 PM and 2:00 AM from patients being evaluated (usually to rule out malaria) at district hospitals (or their equivalent) throughout the country. Areas undersampled by the laboratory-based surveillance system were identified through review of the catchment area map (Figure 2A). Surveillance in those areas was expanded by recruiting a primary care health post (dispensary) in each area to participate in expanded surveillance. One nurse from each dispensary was trained by the MOH staff to collect filter paper blood spots from 20 patients quarterly at each health center from a convenience sample of adult patients presenting for any reason. This number of samples of 20 patients was considered the maximum that was feasible in the setting. After obtaining consent, nurses collect a fingerstick blood sample, which is dried onto filter paper. The dried blood spots are stored in plastic bags at −20°C until collection by the MOH staff, generally quarterly. Then, they are transported to the central MOH laboratory in Lomé, where they are batched for testing by trained laboratory technicians using the Og4C3 enzyme-linked immunosorbent assay (ELISA) testing method (TropBio, Townsville, Queensland, Australia) according to the manufacturer's protocol. All persons testing positive for LF antigenemia by ELISA were subsequently tested by nocturnal thick blood smear using fingerstick capillary blood drawn by trained MOH technicians between 10:00 PM and 2:00 AM.
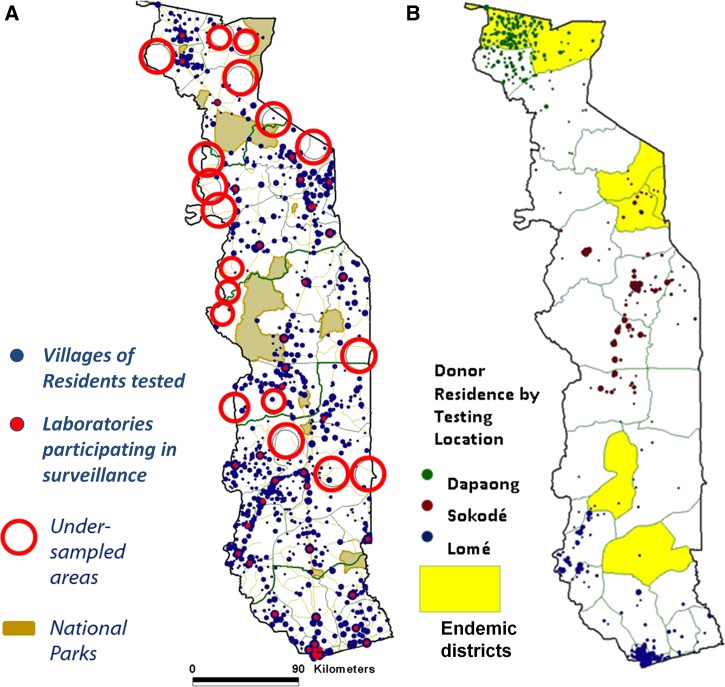
Figure 2.
Coverage of (A) laboratory-based surveillance system (2006–2007) and (B) blood banks in Togo (2010–2011).
Nationwide reassessment of LF transmission status.
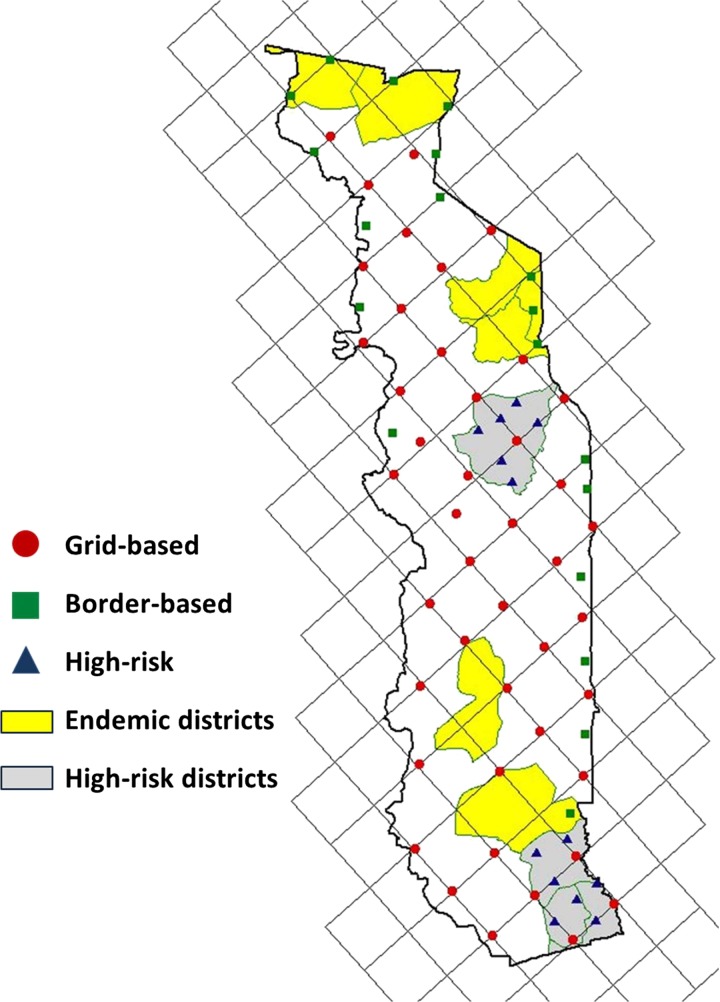
To confirm that the surveillance data truly represented absence of LF transmission in Togo, in 2010 we repeated nationwide mapping using a modified version of the spatial analysis methodology used in the initial mapping of Togo, Benin, and Burkina Faso.10,11 A 35 × 35-km grid was created and randomly oriented over a shapefile of Togo using the Jennessent repeating shapes extension for ArcView 3.x (Figure 3).12 All available village coordinates from the MOH and prior field studies were plotted, and the village nearest each vertex of the grid was selected for inclusion in the survey. The seven LF-endemic districts were excluded from the reassessment survey, because these districts received MDA and therefore were already under monitoring and evaluation.
Figure 3.
Villages selected for reassessment survey in Togo from 2010 to 2011.
The survey intentionally oversampled areas at high risk for LF transmission, which are in districts with historically high lymphedema prevalence. A second grid of 17.5 km × 17.5 km was aligned to the 35-km grid, and additional villages were selected using the same technique as for the larger grid (Figure 3, triangles). Along the national borders with LF-endemic regions of neighboring countries, a 10-km border zone was plotted using the buffer function in ArcView 3. In this buffer, additional villages were selected wherever the grid line running southwest to northeast intersected a border area, and additional villages were selected wherever gaps > 40 km occurred between two selected villages within the border buffer area (Figure 3, squares). Because southeastern Ghana is not LF-endemic,10 oversampling was not performed along the southwestern border with Ghana.
Trained technicians from the MOH tested a convenience sample of 100 adults in each village (usually the first 100 persons to congregate at the home of the village chief or other central meeting place). After obtaining written consent, the technicians collected and tested 100 μL fingerstick capillary blood for W. bancrofti antigenemia using Binax immunochromatography card tests (ICTs; Invereness Medical, Princeton, NJ) according to the manufacturer's protocol. As a quality control measure, all positive tests and 2% of all negative tests were repeated. Persons testing positive on two of two ICT tests were considered true positives. All persons testing positive by ICT were subsequently tested by nocturnal thick blood smear as described above.
Evaluation of other possible LF surveillance systems.
As described above, the laboratory-based surveillance system relies on testing hospitalized patients. To ensure that we were using the best sampling method for collecting LF surveillance data, we also considered the other sampling populations proposed by the WHO: military recruits, students, and blood donors.4,5 After a preliminary review of all options, we collected more information on the most viable alternative—the blood bank. The National Blood Bank in Togo performs centralized testing of donated blood at Togo's three regional centers: Dapaong in the north, Sokodé in the center of the country, and the capitol of Lomé in the south (Figure 2B). From each of these locations, we collected data on age, sex, village of residence, and given profession (where available) of each blood donor over the course of 1 calendar year. Each village of residence was assigned GPS coordinates and plotted using ArcView GIS software as described under mapping of the surveillance system catchment area above.
Ethics.
Protocols for all activities described were evaluated by a CDC human subjects review board and deemed to be programmatic evaluation not involving human subject research. In addition, the transmission reassessment survey received institutional review board approval from the Togolese MOH. All persons tested in the reassessment survey provided written consent; patients whose blood was collected in hospitals or dispensaries, where the primary purpose of the blood collection was generally for clinical indications not related to LF surveillance, provided oral consent before capillary blood collection.
Results
Evaluation of geographic coverage.
The geographic catchment area of the laboratory-based LF surveillance system during the first 2 years of implementation is shown in Figure 2A. The system achieved good geographic coverage, with at least one hospital laboratory from 97% of the districts included. However, few patients residing in areas remote from the surveillance hospital laboratories were tested. Excluding the national parks, we identified 18 geographic areas that were undersampled (Figure 2A, red circles).
Improved surveillance protocol.
From 2010 to 2011, 2,183 blood spots were collected in dispensaries within each of the undersampled areas. None of these blood spots tested positive (Table 1). During the same period, ongoing surveillance at hospital laboratories using nocturnal thick blood smears detected 1 patient among 6,509 patients tested (0.02%) with positive microfilaremia (Table 1).
Table 1.
Results of ongoing LF surveillance in Togo from 2010 to 2011
| Location of sample collection | Surveillance method | Samples anticipated* | Samples tested (% of anticipated) | Samples positive (% of tested) |
|---|---|---|---|---|
| Hospital laboratories | Nocturnal thick blood smear | 9,840 | 6,509 (66) | 1 (0.02) |
| Dispensaries | Filter paper blood spot (ELISA) | 2,880 | 2,183 (76) | 0 (0.00) |
| Total | 12,720 | 8,692 (68) | 1 (0.01) |
The number of samples expected if all participating surveillance locations had submitted 100% of requested samples.
Reassessment of LF transmission status.
In the reassessment survey, 7,800 persons in 78 villages were tested by ICT. Twenty-three persons (0.3%) residing in six separate villages tested positive (Table 2). All ICT positives were then retested by nocturnal blood film, with none showing positive microfilaremia (Table 2). As a control, 156 (2%) of the negative tests were also repeated; none were positive.
Table 2.
Results of nationwide reassessment of LF transmission status in Togo from 2010 to 2011
| Sample group | Villages tested | Villages with at least one ICT positive | Individuals tested | Individuals testing positive by ICT | Individuals confirmed positive by NTBS | ||
|---|---|---|---|---|---|---|---|
| N | Percent | N | Percent | ||||
| Vertices of 35 × 35-km grid | 44 | 6 | 13.6 | 4,400 | 9 | 0.2 | 0 |
| Border zones | 19 | 5 | 26.3 | 1,900 | 14 | 0.7 | 0 |
| Districts with historically high lymphedema prevalence | 15 | 0 | 0.0 | 1,500 | 0 | 0.0 | 0 |
| Total | 78 | 11 | 14.1 | 7,800 | 23 | 0.3 | 0 |
NTBS = nocturnal thick blood smear.
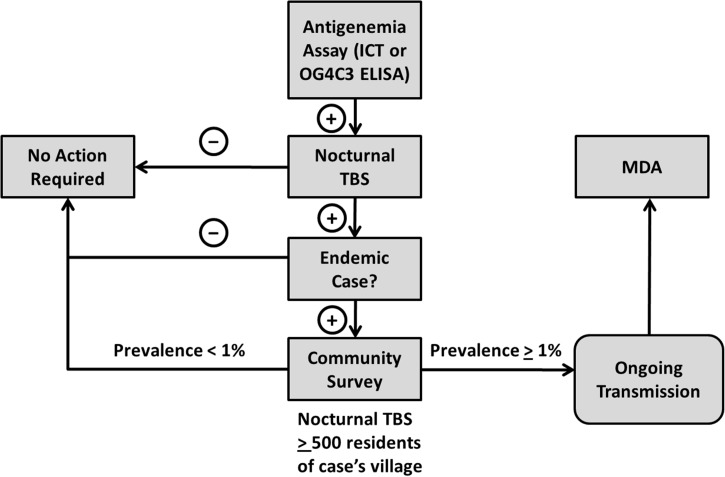
In total, LF surveillance conducted from 2010 to 2011 identified only 1 person positive for microfilaremia among 6,509 people tested by nocturnal blood smear, and 0 of 1,452 people tested by Og4C3 ELISA were positive. The LF-positive person lived in Est-Mono, a district that did not undergo MDA. As per national protocol for follow-up of patients with microfilaremia (Figure 4), a sample of 500 additional persons in her village of residence was tested by ICT.7 None of those individuals tested were positive, and therefore, no additional public health response was initiated.
Figure 4.
Strategy for follow-up of ICT or ELISA positive results in Togo from 2010 to 2011. TBS = thick blood smear.
Assessment of surveillance alternatives.
Two of four WHO-suggested surveillance populations were dismissed as insufficiently representative of the Togo population on preliminary review: (1) military recruits in Togo, who are selected predominantly from the northern regions; and (2) university students, who come predominantly from wealthier, urban families and whose risk for contracting LF is low.
Blood bank donors were considered as a possible alternative surveillance population given the potential convenience of screening samples of donated blood, but it was unclear whether the geographical representation would be adequate. Figure 2 shows the location of the three centralized blood testing centers, and Table 3 summarizes the demographic information of the donors tested. The 16,169 blood donors whose blood samples were screened in either 2008 in Lomé or 2009 in Dapaong and Sokodé were predominantly young men (median age = 23 years, range = 17–69 years). Over 60% of donors for whom data regarding profession was available (those individuals tested in Sokodé or Lomé; comprising roughly 85% of all donors) were students. GPS coordinates were available for the villages or cities of residence of 15,754 (97%) donors; when plotted, these coordinates revealed that, with the exception of the area surrounding Dapaong, blood donors in Togo reside mainly in urban or periurban areas (Figure 2B). The geographic representativeness of the blood bank did not compare favorably with the representativeness of the laboratory-based LF surveillance system (Figure 2).
Table 3.
Demographic information of blood donors in Togo from 2008 to 2009
| Testing center | Year | Number of donors | Age of donors | Male sex | Students | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Percent | Median | Range | N | Percent | N | Percent | ||
| National | |||||||||
| Dapaong | 2009 | 381 | 2.4 | 29 | 18–69 | 349 | 91.6 | n/a | n/a |
| Private | 2009 | 1,892 | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
| Sokodé | 2009 | 4,458 | 27.6 | 21 | 17–59 | 3,644 | 81.7 | 3,151 | 70.7 |
| Lomé | 2008 | 9,438 | 58.4 | 24 | 17–69 | 7,382 | 78.2 | 5,419 | 57.4 |
| Total | 16,169 | 100.0 | 23 | 17–69 | 79.7 | ||||
n/a = data not available.
Discussion
Success in final elimination of LF transmission may depend on adequate surveillance and the ability to respond when cases are detected. Lack of adequate post-intervention surveillance can contribute to reemergence of a disease after it is thought to be eliminated, a scenario that was recently shown with the reemergence of Guinea Worm in Chad.13 Reemergence of LF after presumed elimination would likely have disastrous consequences in terms of donor fatigue and diminished political will, which has been historically seen with malaria14–18 and African trypanosomiasis.19–23
A country can be certified as having eliminated LF if it can show that no transmission is occurring anywhere in the country. The WHO-recommended TAS is a robust tool to decide the end of MDAs and subsequently monitor the absence of transmission after the end of interventions. However, the TAS is required only in districts that undergo MDA, and it is resource-intensive, making it difficult to mobilize for districts declared not endemic after the initial mapping. For this reason, ongoing surveillance may be the only mechanism to detect missed or newly imported foci of LF transmission in areas deemed non-endemic at initial mapping.
Effective post-MDA surveillance must be capable of detecting reemergence of LF transmission in a timely manner and should trigger an appropriate public health response to identified cases. We believe an effective and practical passive surveillance program for LF should incorporate four key principles: (1) adaptation to the needs and capacities of the country, (2) coverage of all at-risk areas, including those areas that may have been initially mapped as non-endemic, (3) implementation before cessation of MDA if possible, and (4) a well thought out response to cases that may be identified through surveillance. What has been learned regarding these principles through years of experience with LF surveillance in Togo is outlined below.
First, it is clear that surveillance must be adapted to the needs and capacities of the country to be viable and sustainable. LF surveillance in Togo has been adapted to the needs and capacities of the country in several ways. It was conceived to take advantage of existing health infrastructure and interventions by using nocturnally prepared thick blood smears from patients presenting to hospitals throughout the country for ruling out of malaria, and it has been further adapted to existing infrastructure through the central laboratory-based testing of filter paper blood spots collected at remote dispensaries. Our experience to date has been that ELISA-based surveillance using blood collected on filter paper is a practical and feasible way of extending surveillance to remote areas without laboratory capacity. Collection of blood spots in remote dispensaries requires only the ability of the staffing nurse to collect a finger prick blood sample and an available freezer, where dried blood spots can be stored before shipping. Pooling of samples and processing in batches at a central laboratory minimize waste. We have found blood spot-based ELISA testing to be a useful complement to the blood slide-based testing performed in hospital laboratories.
Because it is convenient to add LF testing to donated blood screening within the National Blood Bank system, screening donated blood for LF antigenemia is an appealing alternative surveillance strategy at first glance. Unfortunately, we found the blood donor population in Togo to be inadequately representative to justify replacement of the current surveillance system with blood bank-based surveillance, although such a strategy may prove useful elsewhere.
Second, the surveillance system should cover all areas potentially at risk for reemergence of LF transmission and not just those areas that have undergone MDA. Given the possibilities of missed foci at initial mapping and reimportation by nomadic tribes through cross border travel from endemic areas of neighboring countries, surveillance is conducted nationwide in Togo. Because post-MDA monitoring and evaluation activities are limited to implementation units where MDAs were conducted, ongoing passive surveillance is the only mechanism by which areas at risk but deemed non-endemic at initial mapping are monitored. The value of this surveillance strategy in Togo is illustrated by the fact that the three LF cases detected to date (two cases reported previously7 and one case reported here) were all detected in districts categorized as non-endemic by initial mapping.
Third, LF surveillance should be implemented before cessation of MDA whenever possible for several reasons. There is stronger political will to implement a new protocol before cessation of MDA. After MDAs are completed, MOHs with limited resources may wish to reallocate resources away from LF programs, making it more difficult to establish effective surveillance. In addition, if cases are detected by surveillance, it will be easier for national programs to mount an adequate response, including potential implementation of additional MDAs, if the MDA infrastructure has not already been dismantled. Another advantage of implementing LF surveillance concurrently with MDAs is that it may test the sensitivity of the surveillance system; data from both sources may be compared, and modifications to the surveillance system made as necessary.
LF surveillance in Togo has detected only three cases of true microfilaremia of W. bancrofti from 2006 to 2011, and to date, it has detected no evidence of ongoing LF transmission around the cases. Presumably, this finding reflects a lack of ongoing transmission, which was confirmed by the nationwide reassessment survey. Although it is satisfying that the reassessment survey detected no evidence of ongoing LF transmission in Togo, validation of the sensitivity of the surveillance system is not possible in the current environment and will require its implementation in an area of ongoing transmission.
Fourth, an effective surveillance system must include a clear and well-thought out algorithm for responding to cases that might be identified. The purpose of surveillance is to determine whether there is a need for public health action,9 and an effective surveillance system must define the nature of this action and the threshold for triggering it. A schematic of the algorithm used to respond to detected LF cases in Togo is shown in Figure 4. In this system, the trigger for implementation of MDAs is the discovery of > 1% microfilaremia within one community. Microfilaremia rather than antigenemia was chosen as the trigger for public health action, because it is the most immediate indicator of transmission risk.
All surveillance testing for LF in Togo is conducted among adults; a maximally sensitive surveillance system is desired, and adults generally have a higher prevalence of microfilaremia than children. This policy also simplifies informed consent. Unlike the presence of LF antigenemia in a young child, antigenemia or microfilaremia in an adult is not specific for recent LF transmission. However, microfilaremia at any age represents risk for transmission (because circulating microfilariae can be transmitted to biting mosquitoes); therefore, all positive antigenemia tests (ICT or Og4C3 ELISA) are confirmed by nocturnal thick blood smear in Togo. Although the current report focuses on surveillance to ensure interruption of transmission, morbidity control efforts have also been an essential component of the LF control program in Togo and have recently been described elsewhere.6,24 Because hydrocele and lymphedema are poor indicators of active filarial burden,25 they are not currently used for surveillance of LF transmission in Togo; incorporation of these indicators of LF-related morbidity into ongoing surveillance should be considered.
With the expansion and success of the global effort to eliminate LF transmission, more and more endemic countries are nearing cessation of MDA and approaching the period of passive surveillance. Our experience in Togo shows that effective LF surveillance can be implemented in resource-poor settings but that the surveillance must be adapted to the needs and capacities of the country and should be conducted in all at-risk areas of the country (both endemic and non-endemic). Wherever possible, the ongoing surveillance system should be implemented before cessation of MDA. The surveillance strategy chosen should have clear algorithms for the follow-up of any identified cases and predetermined thresholds for the implementation of MDA in areas found to have ongoing LF transmission. We have shown here how these principles (summarized in Table 4) have been developed and integrated into a post-MDA surveillance system in Togo, and we hope that this work may provide a model for implementation of LF surveillance for other countries nearing elimination of LF transmission.
Table 4.
The surveillance system and lessons learned in Togo from 2010 to 2011
| Lessons learned | Practical experience in Togo |
|---|---|
| 1. Adapt to available resources and infrastructure | A combination of clinical laboratory-based testing using blood slides and dispensary-based testing using Og4C3 ELISA is used |
| 2. Cover all areas of the country potentially at risk (not just areas that underwent MDA) | Surveillance conducted nationwide; only three cases were discovered from 2006 to 2011, and all three cases were in districts that did not receive MDA |
| 3. Wherever possible, implement before cessation of MDA | The best opportunity to test the sensitivity of the surveillance system will be before MDA has reduced prevalence to low levels; in addition, momentum (funding and staff) for surveillance may be easier to achieve before cessation of MDA |
| 4. Pre-specify clear triggers for appropriate public health action | Each positive case surveillance result is confirmed by nocturnal blood smear, and each confirmed positive results in a local investigation; the ultimate trigger for reimplementation of MDA is finding a local prevalence of ≥ 1% blood smear positives surrounding an identified case |
ACKNOWLEDGMENTS
This work was made possible by collaboration with many key individuals. We would like to thank the individuals who assisted in data collection activities, particularly Mr. Koffi Otogbe for his assistance in GPS data collection and Dr. Lochina Feteke and his colleagues at the National Blood Bank for enabling the analysis of blood donor data.
Disclaimer: The contents are the responsibility of the authors and do not necessarily reflect the views of the Centers for Disease Control and Prevention and the U.S. Agency for International Development (USAID).
Footnotes
Financial support: This study is made possible by the generous support of the American people through the U.S. Agency for International Development (USAID) and the ENVISION project under cooperative agreement No. AID-OAA-A-11-00048.
Authors' addresses: Philip J. Budge, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, TN, E-mail: Philip.budge@vanderbilt.edu. Ameyo M. Dorkenoo, Program for the Elimination of Lymphatic Filariasis, Ministry of Health, Lomé, Togo, E-mail: monicadork@yahoo.fr. Yao K. Sodahlon, Mectizan Donation Program, Decatur, GA, E-mail: ysodahlon@taskforce.org. Omofolarin B. Fasuyi, Department of Family Medicine, Morehouse School of Medicine, East Point, GA, E-mail: OFasuyi@msm.edu. Els Mathieu, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: emm7@cdc.gov.
References
- 1.Global Programme to Eliminate Lymphatic Filariasis Global Programme to eliminate lymphatic filariasis: progress report on mass drug administration, 2010. Wkly Epidemiol Rec. 2011;86:377–388. [PubMed] [Google Scholar]
- 2.Zeldenryk LM, Gray M, Speare R, Gordon S, Melrose W. The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl Trop Dis. 2011;5:e1366. doi: 10.1371/journal.pntd.0001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHA50 Elimination of Lymphatic Filariasis as a Public Health Problem. 1997. http://www.who.int/neglected_diseases/mediacentre/WHA_50.29_Eng.pdf Available at. Accessed August 27, 2012. [DOI] [PubMed]
- 4.World Health Organization . Monitoring and Epidemiological Assessment of the Programme to Eliminate Lymphatic Filariasis at Implementation Unit Level. Geneva: World Health Organization; 2005. [Google Scholar]
- 5.World Health Organization . Monitoring and Epidemiological Assessment of Mass Drug Administration in the Global Programme to Eliminate Lymphatic Filariasis: A Manual for National Elimination Programmes. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 6.Sodahlon YK, Dorkenoo AM, Morgah K, Nabiliou K, Agbo K, Miller R, Datagni M, Seim A, Mathieu E. A success story: Togo is moving toward becoming the first sub-Saharan African nation to eliminate lymphatic filariasis through mass drug administration and countrywide morbidity alleviation. PLoS Negl Trop Dis. 2013;7:e2080. doi: 10.1371/journal.pntd.0002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu E, Dorkenoo A, Otogbe FK, Budge PJ, Sodahlon YK. A laboratory-based surveillance system for Wuchereria bancrofti in Togo: a practical model for resource-poor settings. Am J Trop Med Hyg. 2011;84:988–993. doi: 10.4269/ajtmh.2011.10-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Guidelines Working Group Centers for Disease Control and Prevention (CDC) Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50((RR-13)):1–35. [PubMed] [Google Scholar]
- 9.Centers for Disease Control (CDC) Guidelines for evaluating surveillance systems. MMWR Morb Mortal Wkly Rep. 1988;37((Suppl 5)):1–18. [PubMed] [Google Scholar]
- 10.Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, Owusu-Banahene G, Sanou S, Sodahlon YK, Biswas G, Kale OO, Molyneux DH, Roungou JB, Thomson MC, Remme J. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol. 2002;96:695–705. doi: 10.1179/000349802125001735. [DOI] [PubMed] [Google Scholar]
- 11.Gyapong JO, Remme JH. The use of grid sampling methodology for rapid assessment of the distribution of bancroftian filariasis. Trans R Soc Trop Med Hyg. 2001;95:681–686. doi: 10.1016/s0035-9203(01)90115-4. [DOI] [PubMed] [Google Scholar]
- 12.Jenness Enterprises Repeating Shapes (repeat_shapes.avx) Extension for ArcView 3.x. 2005. http://www.jennessent.com/arcview/arcview_extensions.htm Available at. Accessed March 1, 2010.
- 13.Centers for Disease Control and Prevention (CDC) Renewed transmission of dracunculiasis—Chad, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:744–748. [PubMed] [Google Scholar]
- 14.Breman JG, Brandling-Bennett AD. The challenge of malaria eradication in the twenty-first century: research linked to operations is the key. Vaccine. 2011;29((Suppl 4)):D97–D103. doi: 10.1016/j.vaccine.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood B. Can malaria be eliminated? Trans R Soc Trop Med Hyg. 2009;103((Suppl 1)):S2–S5. doi: 10.1016/j.trstmh.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH. From malaria control to eradication: the WHO perspective. Trop Med Int Health. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- 19.Cattand P, Jannin J, Lucas P. Sleeping sickness surveillance: an essential step towards elimination. Trop Med Int Health. 2001;6:348–361. doi: 10.1046/j.1365-3156.2001.00669.x. [DOI] [PubMed] [Google Scholar]
- 20.Courtin F, Jamonneau V, Duvallet G, Garcia A, Coulibaly B, Doumenge JP, Cuny G, Solano P. Sleeping sickness in West Africa (1906–2006): changes in spatial repartition and lessons from the past. Trop Med Int Health. 2008;13:334–344. doi: 10.1111/j.1365-3156.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 21.Molyneux D, Ndung'u J, Maudlin I. Controlling sleeping sickness—“when will they ever learn?”. PLoS Negl Trop Dis. 2010;4:e609. doi: 10.1371/journal.pntd.0000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore A, Richer M. Re-emergence of epidemic sleeping sickness in southern Sudan. Trop Med Int Health. 2001;6:342–347. doi: 10.1046/j.1365-3156.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Nieuwenhove S, Betu-Ku-Mesu VK, Diabakana PM, Declercq J, Bilenge CM. Sleeping sickness resurgence in the DRC: the past decade. Trop Med Int Health. 2001;6:335–341. doi: 10.1046/j.1365-3156.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 24.Mathieu E, Dorkenoo AM, Datagni M, Cantey PT, Morgah K, Harvey K, Ziperstein J, Drexler N, Chapleau G, Sodahlon Y. It is possible: availability of lymphedema case management in each health facility in Togo. Program description, evaluation, and lessons learned. Am J Trop Med Hyg. 2013;89:16–22. doi: 10.4269/ajtmh.12-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigege A, Richards FO, Jr, Blaney DD, Miri ES, Gontor I, Ogah G, Umaru J, Jinadu MY, Mathai W, Amadiegwu S, Hopkins DR. Rapid assessment for lymphatic filariasis in central Nigeria: a comparison of the immunochromatographic card test and hydrocele rates in an area of high endemicity. Am J Trop Med Hyg. 2003;68:643–646. [PubMed] [Google Scholar]