Figure 2.
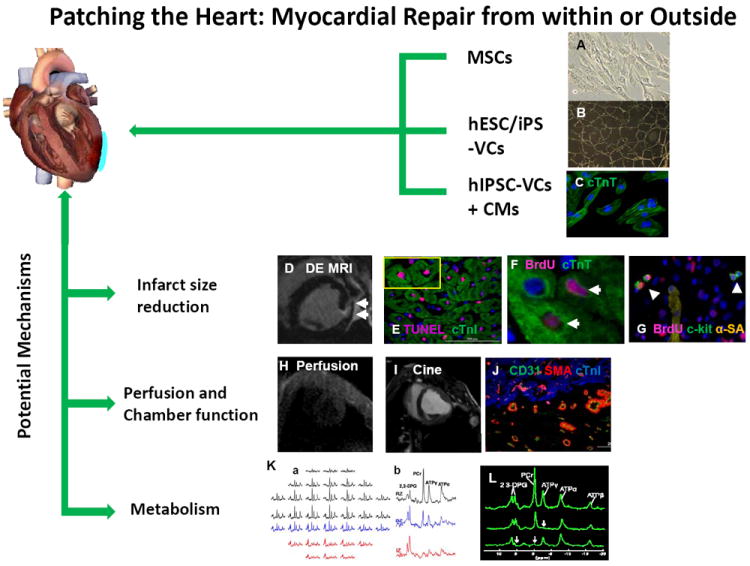
The benefits associated with our in vivo method for cardiac patch application. A circular 3D porous biodegradable cardiac patch (blue) is created over the infarcted region by mixing thrombin and fibrinogen solutions that contain different progenitor cell types, such as mesenchymal stem cells (MSCs), vascular cells (VCs) generated through the controlled differentiation of either human embryonic stem cells (hESCs) or human induced pluripotent stem cells (hiPSCs), or both hiPSC-VCs and cardiomyocytes (hiPSC-CMs). The fibrinogen can be modified to bind peptides for different purposes, such as guiding differentiation or impeding apoptosis. The solution typically solidifies in less than one minute to form a 3D porous cardiac patch that provides structural support as well as a platform for the transplanted progenitor cells, and ultimately increases the cell engraftment rate. The transplanted cells release growth factors and other cytokines that reduce apoptosis, promote angiogenesis, and activate endogenous mechanisms for cardiomyocyte renewal, which leads to declines in infarct size and to improvements in myocardial perfusion, metabolism, and contractile function. Measurements of in vivo myocardial bioenergetics and the ATP turnover rate (via 31P magnetization saturation transfer) suggest that the patch also protects against adverse changes in cardiomyocyte energy metabolism, perhaps by reducing wall stress and bulging at the site of the infarction. Collectively, these benefits improve cardiac contractile function and impede the progression of LV dilatation. (Panels B and K courtesy of Xiong Q et al Circ Res. 2012 ;111(4):455-68; Panels F, G, I, J and L, courtesy of Xiong Q et al Circulation 2013; 127(9): 997-1008).
