Abstract
Percutaneous coronary intervention (PCI) is an integral part of the treatment of coronary artery disease. The most common complication of PCI, bleeding, typically occurs at the vascular access site and is associated with short-term and long-term morbidity and mortality. Periprocedural bleeding also represents the primary safety concern of concomitant antithrombotic therapies essential for PCI success. Use of radial access for PCI reduces procedural bleeding and hence may change the risk profile and net clinical benefit of these drugs. This new drug-device safety interaction creates opportunities to advance the safe and effective use of antithrombotic agents during PCI. In June 2010 and March 2011, leaders from government, academia, professional societies, device manufacturing, and pharmaceutical industries convened for 2 think tank meetings. Titled TREAT I and II, these forums examined approaches to improve the overall safety of PCI by optimizing strategies for antithrombotic drug use and radial artery access. This article summarizes the content and proceedings of these sessions.
Today we are at a critical moment for innovation. Despite the struggling U.S. economy, American pharmaceutical, biotech[nology], and medical device companies have maintained a competitive advantage in the global marketplace... but there are signs that the health of these industries may be at increasing risk...Today's medical product pipeline does not have enough new products to sustain the health of the industry in the long-term to address many of the chronic diseases afflicting Americans every day... We must act now and work together to capitalize on... groundbreaking science in order to bring safer and more effective treatments to American families and keep our position as the global leader in scientific innovation.
- Dr Margaret Hamburg, Commissioner of the Federal Food and Drug Administration (FDA), “A Note from the Commissioner,” Driving Biomedical Innovation: Initiatives for Improving Products for Patients, October 5, 2011.
Safety is a global priority for the development of drugs and devices. This emphasis represents not only interest in optimizing patient outcomes but also recognition of safety concerns as a major barrier to product innovation, development, use, and marketing. Over the last 40 years, more than 120 compounds were withdrawn from the market and from drug development programs worldwide.1-3 Growing pressures to identify rare but catastrophic clinical events affecting “off-target” organ systems have inflated development costs for new therapeutics, and mounting demands for safety demonstration siphon resources from discovery and innovation. The detailed and prolonged assessments for cardiac safety have contributed to the decline in a number of new therapies approved in the United States, despite record investments in biomedical research.4
In 2004, the US FDA introduced its Critical Path Initiative to address the widening gap between scientific discoveries and their translation into medical products. Because concerns about cardiovascular safety can challenge medical product innovation, the Duke Clinical Research Institute (DCRI), Durham, NC, entered into a memorandum of understanding with the FDA to form the Cardiac Safety Research Consortium (CSRC) (www.cardiac-safety.org), a transparent Critical Path public-private partnership of representatives from government, industry, and academia. The CSRC was created to support investigation of cardiovascular safety to facilitate cardiac safety–related regulatory process improvements. One of the key mechanisms through which the CSRC functions is the coordination of think tank, or public forums that promote cross-sector scientific collaboration as well as sharing of data and expertise regarding challenging topics in cardiac safety. These think tanks have produced research projects focused on removing barriers to innovation, benefiting collaborating partners and the public health.5,6
Bleeding associated with adjunctive drugs in the setting of percutaneous coronary intervention (PCI) is a consistently challenging aspect of cardiovascular safety. Percutaneous coronary intervention is an integral part of the treatment of coronary artery disease, improving mortality in acute coronary syndromes and relieving ischemic symptoms in patients with stable coronary artery disease.7 According to market estimates, each year more than 1 million patients in the United States undergo PCI, with approximately 600,000 of these procedures performed in the inpatient setting.8 Intracoronary stents are frequently implanted during PCI to reduce the risk of abrupt vessel closure and the need for repeat procedures. Stents are inherently prothrombotic until fully endothelialized and require short- and long-term concomitant antithrombotic therapies, such as glycoprotein IIb/IIIa inhibitors and dual-antiplatelet therapy, to prevent stent thrombosis. Although largely effective, these therapies also cause bleeding. Procedural success reflects interactions among patient, drug, and device, and the clinical benefit of antithrombotic regimens may be offset primarily by bleeding events, particularly those related to vascular access site complications.
To address the issue of antithrombotic therapy and bleeding complications associated with PCI, 2 think tank meetings, titled TREAT I and II, were organized at the FDA headquarters in White Oak, MD, on June 23, 2010, and March 7, 2011. These forums were the result of collaboration among the CSRC, the DCRI, the American College of Cardiology Foundation (ACCF), the Society for Cardiac Angiography and Interventions (SCAI), the National Heart Lung Blood Institute, the FDA, and national and international representatives from academia, device manufacturing, and pharmaceutical industries (see the online Appendix). The focus of these think tanks was to reevaluate optimal antithrombotic strategies in light of reduced PCI bleeding risk associated with contemporary radial vascular access. Participants from TREAT explored the “obligatory drug-device safety interaction” paradigm by reversing the traditional perspective of using drugs to enhance procedural safety into a novel view in which modification of the procedural approach improves the safety and net clinical benefit of antithrombotic therapies. Participants examined strategies for demonstrating whether radial artery access changes the optimal risk-benefit ratio for currently recommended anticoagulant and antiplatelet strategies. The potential effects of transradial PCI on patient care and hospital resource use, as well as barriers to the widespread adoption of the radial technique, were also examined. This white paper summarizes the content and proceedings of the TREAT think tank discussions.
Bleeding and PCI: an overview
Although definitions of bleeding vary among reports, periprocedural bleeding is a common complication after PCI, with estimates of the incidence of major bleeding after PCI ranging from 2.2% to 14%.9-12 Use of antithrombotic agents, important adjuncts to successful PCI, has been limited by this bleeding.13,14 Overall rates of bleeding vary among drugs, but vascular site bleeds represent an important proportion of all bleeding events.15 Kinnaird et al10 performed a retrospective analysis of 12,029 PCIs and found 588 major and 1,394 minor bleeding events, of which 68% and 60%, respectively, were related to vascular access in the form of hematomas or retroperitoneal bleeds. Data from pooled clinical trials found that 925 (5.3%) of 17,393 patients undergoing PCI experienced a bleeding event classified as Thrombolysis in Myocar-dial Infarction major/minor bleeding. Of these bleeding events, 54.3% involved the access site, 15.4% were from non–access sites, and 30.4% were from unidentified sites.16
Percutaneous coronary intervention–related bleeding complications have important short- and long-term consequences. Short-term consequences include prolonged hospital stay, need for blood transfusions and surgical intervention, and interruption of antithrombotic therapy otherwise needed for prevention of stent thrombosis and secondary ischemic events. The long-term sequelae of bleeding include major adverse cardiac events, although a causal relationship between bleeding and such outcomes has not been elucidated.10,17-20 Recent analysis of data from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial showed that patients with major bleeding had significantly higher rates of 30-day mortality (7.3% vs 1.2%, P < .0001), composite ischemia (defined as death, myocardial infarction [MI], or unplanned revascularization for ischemia; 23.1% vs 6.8%, P < .0001), and stent thrombosis (3.4% vs 0.6%, P < .0001) compared with patients without bleeding.17 Ndrepepa et al20 examined periprocedural bleeding in 4 randomized clinical trials (RCTs) of the platelet glycoprotein IIb/IIIa receptor inhibitor abxicimab and found that the 30-day occurrence of bleeding was an independent predictor of 1-year mortality (hazard ratio [HR] 2.96, 95% CI 1.96-4.48, P < .001). Furthermore, both non–access site and access site bleeding after PCI are associated with mortality; there is an approximately 2-fold increase in risk of 1-year mortality associated with non–access site vs access site bleeding (HR 3.94 [95% CI 3.07-5.15, P < .0001] vs HR 1.82 [95% CI 1.17-2.83, P = .008]).16 Although there is a stronger association of non–access site bleeding with mortality, reductions in overall bleeding risk and access site bleeding specifically remain important targets for improving PCI outcomes.
Vascular access safety in women undergoing PCI
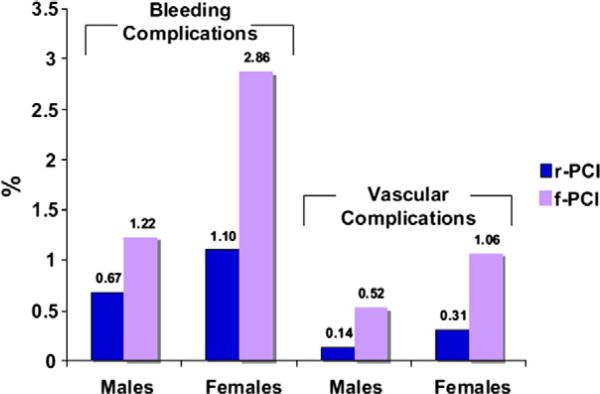
Women undergoing PCI are at increased risk for antithrombotic drug-related bleeding and vascular complications. Women have higher rates of bleeding than men after PCI,21,22 and multiple studies show that female sex independently predicts bleeding and death after PCI.23,24 Although overdosing of antithrombotic medications theoretically contributes to the increased bleeding experienced by women after PCI, data suggest that excessive medication accounts for only 25% of the sex-related difference in bleeding risk.25 In addition to higher rates of post-PCI bleeding, women also have more frequent access site complications, regardless of access site and use of vascular closure devices (Figure 1).26-31 Despite available bleeding avoidance strategies, there exists a “risk-treatment paradox.” Data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry have shown that use of vascular closure devices and antithrombotic medications with lower bleeding risk, such as bivalirudin, are used less often in patients at higher risk for bleeding, including women.32
Figure 1.
Bleeding and vascular complications after PCI in men vs women. rPCI, radial PCI; f-PCI, femoral PCI. From Rao et al.21
An overview of transradial PCI
The radial artery is small, superficial, easily compressible, and free of neighboring major structures, making vascular complications and bleeding less frequent and more easily managed than with femoral artery access. Randomized trials such as RadIal versus femorAL access for coronary angiography or intervention in patients with acute coronary syndromes (RIVAL) have shown that the radial approach is associated with reduced bleeding and vascular complication rates compared with femoral access.33-35 In a large observational study from the NCDR, Rao et al21 examined 593,094 PCI procedures and found that 0.19% of patients undergoing radial access vs 0.70% of patients undergoing femoral access had vascular complications. Additional benefits of transradial intervention (TRI) include faster ambulation, greater comfort, and shorter hospital length of stay. A small, randomized trial also found that TRI improved quality of life and was preferred by patients.36
Advantages of transradial access may also extend to longer-term outcomes. The Mortality benefit Of Reduced Transfusion after PCIs via the Arm or Leg (MORTAL) study found that among 38,872 PCI procedures, radial vs femoral access was associated with reduced rates of blood transfusion (odds ratio [OR] 0.59, 95% CI 0.48-0.73) and 30-day and 1-year mortality (OR 0.71 [95% CI 0.61-0.82] and OR 0.83 [95% CI 0.71-0.98], respectively).37 Other randomized trials including RIVAL have not confirmed this mortality reduction associated with radial access.
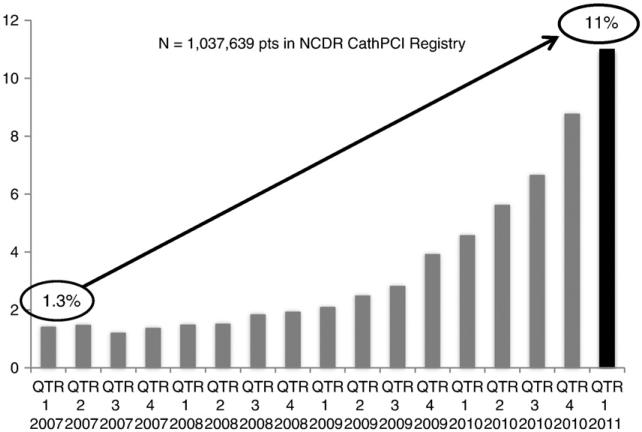
Despite several advantages offered by a transradial approach, adoption of this technology has been relatively slow in the United States. In 2007, TRI accounted for only 1.32% of procedures.21 As shown in Figure 2, recent data show that the use of radial access has increased to 11% of PCIs (personal communication, NCDR) but still accounts for a small minority of procedures. Difficulties with radial access and the documented “learning curve” for TRI are important potential barriers to US uptake.38 The smaller radial artery may not accommodate interventional catheters as easily as the femoral artery, and spasm, tortuosity, and occlusion of the radial artery pose additional challenges. Compared with femoral procedures, radial procedures have been associated with significantly increased rates of procedural failure, access site crossover, longer procedure times, greater use of contrast, and higher radiation exposure.33,39 However, additional operator experience and movement along the learning curve promote significant reductions in procedural time and radiation dose,15,40 with a decrease in the odds of procedural failure of 32% for each 50 case increase in PCI volume.41 Despite these data, some TRI operators remain discouraged by initial technical difficulties and logistical challenges of converting catheterization laboratories accustomed to femoral PCIs because hospital environments may not have necessary equipment, and staff may initially lack knowledge regarding radial procedure setup and patient care. In these settings, low operator or hospital procedure volume may limit the adoption of a new technique.
Figure 2.
Temporal trends in use of radial PCI in the United States since 2007. Data courtesy of NCDR. QTR, quarter.
Non-procedural factors have also been cited to explain the lack of US adoption of radial artery access. One of the most often cited explanations has been the deficiency in transradial exposure during fellowship training. There are no general guidelines for TRI training and, specifically, no minimal volume requirements for competence. Moreover, radial artery access was only recently included for the first time in the ACCF/American Heart Association/SCAI guidelines for PCI as a class IIa (level of evidence: A) recommendation in the November 2011 update.7 The paucity of radial vs femoral RCTs with US enrollment may also contribute to both the reluctance to adopt TRI and its relatively late inclusion in practice guidelines. Conversely, the lack of US operator experience may limit feasibility of domestic RCTs of bleeding rates related to vascular access and concomitant drugs. There are several approaches to address this important scientific gap.
A national transradial registry?
The FDA has recognized that most efficacy trials of antithrombotic agents predated routine performance of TRI, and hence, bleeding rates of drugs used for femoral PCI procedures might overestimate rates of TRI-associated bleeding. As a result, the FDA initially expressed interest in a national transradial registry to compare different antithrombotic and vascular access strategies to inform drug labeling. Such a database could use the NCDR's CathPCI Registry as a backbone to permit rapid broad sampling of “real- world” clinical practice across variations in antithrombotic treatment strategies. Designing nested studies within the registry could also allow investigators or manufacturers to prospectively address questions regarding potential interactions among patient factors, drug regimens, vascular access sites, and bleeding rates. However, there were concerns that systematic differences in drug regimens between transradial and femoral interventions might confound comparisons between registry patients and historical controls. Observational data would likely be insufficient to inform drug safety and would be limited in the amount of longitudinal data that could be collected, motivating interest in RCTs of vascular access with close attention to concomitant drug therapy.
Extending collaboration and efficiency through infrastructure: CSRC joins with the NCRI
Discussion about ways to foster efficiency using existing registry infrastructure for a prospective RCT centered on the National Cardiovascular Research Infrastructure (NCRI). The DCRI and the ACCF have collaborated to develop a clinical research network to allow investigators to take advantage of the data collection of the American College of Cardiology's NCDR registries. The NCRI has been tasked with maintaining this centralized research network. Multiple uses of such a powerful research instrument include site profiling and recruitment, integrated randomization mechanisms, site training, and quality improvement and education processes. Data elements use standardized definitions, and data are entered once by sites with direct electronic transfer to the clinical trial database for analysis. Designing and executing a prospective, randomized study of vascular access using such a collaborative research network would have many advantages such as reduced work for site coordinators and enhanced quality of patient data.
Radial PCI study design: key issues and lessons from RIVAL
TREAT considered several key issues in designing a study of radial vs femoral PCI. One proposed study design involved concomitant enrollment of patients in select dedicated femoral and radial access sites. Comparative statistical analyses would then be conducted on case-matched patients. However, such a study would not provide sufficient randomized data to inform drug safety. Instead, consensus was reached that a RCT was preferable.
Organizational lessons from the international RIVAL trial were discussed with regard to designing a US-based RCT of vascular access.35 In RIVAL, finding sites and operators with equipoise suitable for randomization was challenging. Some experienced radialists with very high (>90%) radial volume and significantly improved bleeding outcomes were strong advocates of the benefits of TRI and considered randomization to femoral access unethical. Patients and catheterization laboratory staff at high-radial-volume centers similarly exhibited a strong preference for radial access and often refused randomization to a femoral approach. Conversely, sites with operators using predominantly femoral access did not have technical equipoise to randomize to radial access due to inexperience and the early learning curve. The advantage of using only expert, high-volume operators, and dedicated sites would be the testing of hypotheses using optimal practices. However, the generalizability of these findings would be limited because they would be applicable to a small, select group of US operators and would not account for the learning curve. High-volume operators may also use different intraprocedural antithrombotic regimens than femoral operators, thus confounding observed bleeding rates.
The site selection strategy in RIVAL targeted sites in transition from femoral to radial access. The main advantage of this approach was the avoidance of both the learning curve for high-volume femoralists and the lack of clinical equipoise with high-volume radialists. Disadvantages of this strategy might be higher crossover rates, reduced statistical power to detect differences between the 2 approaches on an intent-to-treat basis, and the potential to underestimate the benefits of one approach over the other. Indeed, the results of RIVAL, which were not available during the TREAT sessions, showed no difference with respect to the primary composite end point of death, MI, stroke, and noncoronary artery bypass graft surgery–related major bleeding at 30 days.35
Optimally, an RCT of vascular access would be focused on a question for which there is clinical equipoise such that experienced radial and femoral operators would be willing to participate. Such a design would permit observations about the potential impact of the radial approach in the hands of its most experienced operators. In addition, results from this study could help to provide benchmark metrics such as procedure times, contrast volume, and radiation dose, which could be useful for best-practice guidelines and the evaluation of training methods. Although this trial design would ideally favor inclusion of high-volume operators in experienced centers, reliable identification of these expert operators/centers in the United States would be difficult: operator and site volume for radial procedures are not always correlated because many experienced radialists in the country constitute a minority of practitioners at their sites. The NCDR's CathPCI Registry was suggested as a tool to identify potential trial operator and site participants through its ability to profile radial PCI volume. An added benefit of this approach would be the site's familiarity with the NCDR case report form (CRF) that would serve as the basis for electronic trial data collection.
Bleeding was chosen as one of the primary outcomes for the proposed transradial RCT, but bleeding definitions remain controversial. Multiple definitions of bleeding based on both clinical events and laboratory parameters currently exist and have been used inconsistently in clinical trials including pivotal registration studies. The recently published Bleeding Academic Research Consortium (BARC) definitions were developed to counter such heterogeneity.42 There was consensus agreement that the primary bleeding end point for a radial vs femoral access RCT should incorporate these definitions.
Nonbleeding outcomes were also recognized as key end points to capture. Evaluation of procedural metrics including length of procedure, radiation dose, contrast volume, and procedural success would allow for valuable comparisons between vascular access approaches. Collecting specific drug information instead of general classes of antithrombotic agents would provide the ability to assess the safety profiles of individual medications. Meeting attendees also wanted to examine the impact of radial access on short-term quality of life through earlier ambulation and less discomfort, as well as hospital resource use through reduced length of hospital stay. Additional trial end points would include effects of vascular complications on long-term outcomes and quality of life, such as residual arm discomfort and radial arterial occlusion. However, specific instruments to measure these quality of life effects currently do not exist and need to be developed and validated.
The inclusion of patients presenting with ST-segment elevation MI (STEMI) in a trial of radial vs femoral access for PCI was debated. Operators from experienced radial centers outside the US cited routine use of radial access for STEMI cases and clinical benefit of such a vascular approach in this population.43 However, the generalizability of these observations to US centers was thought to be limited because the lack of widespread radial operator expertise and catheterization laboratory staff experience in the United States might compromise STEMI door-to-balloon times and patient outcomes. The final decision was thus made to exclude patients with STEMI from an RCT of vascular access. However, data from multiple studies suggest that TRI used for STEMI may be associated with clinical benefit, raising the possibility of revisiting this issue in the future.44-46
Identifying clinical equipoise: vascular access in women
The concept of clinical equipoise was fundamental to the identification of an appropriate study population for a randomized trial of vascular access. All agreed that women would be the one cohort for which this trial could be conducted with clinical equipoise (Table I). Women have a greater risk of bleeding, particularly from the access site, but also have smaller arteries that are more prone to spasm. Thus, the selection of optimal vascular access strategy in this group remains unclear; the potential for women to benefit from less bleeding with TRI could be offset by an increase in procedural failure or vascular complications. For these reasons, some transra-dial interventionalists were more willing to randomize women to assess the net clinical benefit of TRI in this high-risk population. Along with the decision to use women as the study population came recognition of the need to capture data relevant to the evaluation and treatment of cardiovascular disease in women. Such information would include menopausal status, use of hormone replacement therapy, and a specific history of vasospastic or autoimmune diseases, as well as measures of functional loss or debility following PCI.
Table I.
Clinical equipoise for a RCT of vascular access in women
| Favors radial approach | Favors femoral approach |
|---|---|
| • Females are at higher risk for bleeding after PCI21 | • Females have smaller radial arteries, which may contribute to procedural failure (more prone to spasm, may not accommodate 6F systems) |
| • Females are at higher risk for vascular complications after PCI21 | • Females have higher rates of radial artery occlusion47 |
| • Females are more likely to receive overdosing of antithrombotic medications25 | • Females have higher rates of hematoma after radial PCI31 |
Study of Access Site for Enhancement of PCI for Women
The Study of Access Site for Enhancement of PCI for Women (SAFE-PCI for Women) trial, a proof of concept study, was the result of a series of medical device safety and collaborative research initiatives, including CSRC Critical Path think tank (Figure 3), that led to the development of a study that addresses an important clinical question and leverages unique trial infrastructure. The trial was designed to be the first randomized study comparing the transradial to transfemoral approach PCI with a focus specifically on women's health. The SAFE-PCI trial also represents the first use of the NCRI clinical research network and the initial application of the BARC bleeding definitions. Through recommendations for contemporary procedural medication regimens, including use of bivalirudin and P2Y12 inhibitors, the investigators aim to determine clinically important outcomes in best-practice scenarios.
Figure 3.
Timeline of events.
The SAFE-PCI study population will include women undergoing elective PCI or presenting with non–ST-segment elevation acute coronary syndromes. Patients will be randomized to radial or femoral access and will have their procedures performed by operators experienced in the assigned vascular approach. Data from the NCDR CathPCI Registry will be used for site identification, CRF development, and data entry through automatic transfer of information entered into CathPCI to the trial CRF. The primary end points for SAFE-PCI will be bleeding according to BARC criteria (types 2, 3, or 5 bleeding)42 and vascular complications (arteriovenous fistula, pseudoaneurysm, or arterial occlusion requiring surgical intervention) at 72 hours postprocedure or by hospital discharge, as well as procedural failure. Secondary end points for the trial will include assessments of procedural metrics, quality of life, and the 30-day occurrence of death, vascular complications, or unplanned revascularization. Full details of SAFE-PCI will be forthcoming in a separate article.
Beyond SAFE-PCI
The SAFE-PCI for Women study represents one of several new directions for conducting research on vascular access safety and for expanding uptake of TRI in the United States. Using highly experienced radial operators in SAFE-PCI will provide some performance metrics and standards that can potentially be used as benchmarks for quantifying progress along the learning curve and measuring the impact of educational programs and other tools such as simulators. The potential for global participation in TREAT programs could leverage the far greater TRI experience outside the United States. Transradial intervention uptake in the United States could also be aided by making available training courses and Web sites for established interventionalists interested in adding TRI to their repertoire and by exposing new interventional cardiology fellows to transradial catheterization during the early stages of their training. To this end, one mechanism to achieve the latter goal might be to revise and expand competency requirements specifically for TRI in the ACCF/SCAI Core Cardiology Training Symposium guidelines. In addition, promoting a team approach that educates interventionalists, catheterization laboratory staff, and referring physicians about TRI and that emphasizes the overlap in use of standard catheterization equipment for both femoral and radial PCI will be important in easing the transition of primarily femoral laboratories to TRI.
Further innovation will be required to overcome the barriers to research posed by the ongoing focus on cardiac safety. A number of pragmatic approaches to address these obstacles have already been implemented, and renewed federal interest in cardiac safety is evident in the many grants awarded as part of the American Recovery and Reinvestment Act for comparative effectiveness research. Efforts have been made to streamline and ensure the accuracy of data entry and transfer through the adoption of electronic health records, computerized databases, and standardized data definitions. Increasing use of data from registries and linkage of databases has also allowed for longitudinal assessment of outcomes. Importantly, emphasis has been placed on the roles of professional societies and academia for ensuring cardiac safety. Physician members of these organizations not only advocate for their patients but also develop practice guidelines, accreditation standards, and policy documents, and they serve on drug safety monitoring boards and clinical events committees. Professional societies and academic institutions additionally provide independent data collection and analysis. To these efforts, the SAFE-PCI study, a randomized, controlled clinical trial nested within an existing electronic data registry, will now add a new paradigm for conducting patient-oriented research. This more efficient and less costly design may have implications for fundamentally changing the construct of US-based clinical research.
Conclusions
In response to the current landscape of barriers to biomedical research, the CSRC was launched to focus on cardiac safety and foster innovation by overcoming precompetitive barriers through transparent dialogue and novel, collaborative research programs. An important operational tool for the CSRC is the organization of think tank meetings comprised of representatives from government, industry, and academia dedicated to addressing specific issues related to cardiac safety of medical products. In one area of such CSRC activities, the domain of drug-device safety interactions, the opposing risks of thrombosis and bleeding associated with PCI procedures are central to overall cardiovascular safety. The potential to change device technique to inform drug safety labeling for antithrombotic compounds represents a novel area of obligatory drug-device safety interactions. As illustrated in this white paper, the CSRC has facilitated important dialogue leading to the creation of a novel RCT, SAFE-PCI, that will not only answer important clinical questions in women's health but also serve as a proof of concept for using efficient infrastructure and electronic data capture as a new paradigm for US clinical research. Efforts such as these are in line with Commissioner Hamburg's vision for restructuring the nation's current framework for biomedical innovation.
We know that the constant developments in science and technology—in fields as diverse as genomics and nanotechnology—hold the promise of major therapeutic advances. But, right now, we lack the ability to effectively translate many of those developments into vital products for those who need them...FDA can help make trials more efficient by qualifying biomarkers that accurately predict outcomes and by encouraging innovative trial designs that are equally effective but less burdensome and time-consuming... Our success will depend on out-reach and collaboration. We must be active participants in research and development through partnerships with academia, industry and other government agencies.
- Dr Margaret Hamburg, Remarks at the American Association for the Advancement of Science Forum on Science and Technology Policy, May 14, 2010.
Acknowledegments
We thank Drs Morton J. Kern, Venugopal Menon, Tejas Patel, Jon R. Resar, Tara A. Ryan, Jeffrey E. Shuren, Norman Stockbridge, and Jean-François Tanguay for their reviews of this manuscript. C.N.H. was funded by NIH grant 5T32HL069749-09.
Appendix
Participants from Academia and Clinical Practice: Alexandre Abizaid, MD, PhD; Kevin J. Anstrom, PhD; Olivier F. Bertrand, MD, PhD; David Cohen, MD; Donald Cutlip, MD; Connie N. Hess, MD; David R. Holmes, MD; Alice K. Jacobs, MD; Sanjit Jolly, MD; Prashant Kaul, MD; Spencer King, MD; David F. Kong, MD; Mitchell W. Krucoff, MD; Venu Menon, MD; John Messenger, MD; Julie M. Miller, MD; L. Kristin Newby, MD, MHS; Kari Niemelae, MD, PhD; Sharon-Lise Normand, PhD; Tejas M. Patel, MD, DM; Eric D. Peterson, MD, MPH; John Petersen, II, MD, MHS; Sunil V. Rao, MD; Jon R. Resar, MD; George Revtyak, MD; Shigeru Saito, MD; Jean-François Tanguay, MD; Ron Waksman, MD; Stephen D. Wiviott, MD; Chuntao Wu, MD, PhD.
Participants from the US FDA: Daniel Canos, MPH; Hesha Duggirala, PhD; Benjamin C. Eloff, PhD; Emmanuel Fadiran, PhD; Andrew Farb, MD; Andrea Furia-Helms, MPH; Beverly Gallauresi, RN, MPH; John Laschinger, MD; Danica Marinac-Dabic, MD, PhD; Ameeta Parekh, PhD; Ellen Pinnow, MS; Synim N. Rivers, MPH; M. Marius Rozek; Tara A. Ryan, MD, MS, MBA; Vicki Seyfert-Margolis, PhD; Jeffrey Shuren, MD, JD; Mary R. South-worth, PharmD; Norman Stockbridge, MD, PhD; Dale R. Tavris, MD, MPH; Douglas Throckmorton, MD; Shaokui Wei, MD, MPH; Xu (Sherry) Yan, PhD.
Participants from the American College of Cardiology: Ralph Brindis, MD; ToniAnn Cox, BA; Kathleen Hewitt, MSN; Dana Pinchotti; Laura Ritzenthaler; Thomas T. Tsai MD, MSc; Janet S. Wright.
Participants from the European Society of Cardiology: Jean-Philippe Collet, MD.
Participants from Society for Cardiovascular Angioplasty and Interventions: Ian C. Gilchrist, MD; Joel Harder, MBA.
Participants from Cardiovascular Research Foundation: Roxana Mehran, MD.
Participants from Industry: Gary Clifton, MBA; Sidney Cohen, MD; William Daley MD; Markus Dietrich, MD, PhD; Gina Eagle, MD; Mark B. Effron, MD; John Finkle, MD; Ruchira Glaser MD; Jerri Anne Johnson; Dina Justice; Stuart Keller; Matthew Killeen PhD; Patricia Knipper; Janine Lane; Jenny Lim, PhD; Roberto Lovick, PharmD; Tom Mahoney; Clive Meanwell, MD, PhD; Lorenz Muller; Funmi Oduolowu, PharmD; Charles Oh; Margaret Quattrocchi; James Rushworth; David R. Rutledge, PharmD; Anne Vosatka, MD, PhD; Mona M. Wahba; Govinda Weerakkody, PhD; Roseann M. White, MA; Kenneth J. Winters, MD; Theressa Wright, MD; Marjorie Zettler, PhD, MPH.
Participants from the Coordinating Staff: Britt Barham; Lauren Beller; Laura Melton, PhD; Valarie Morrow, MD; RhaShawn Wells; Willette Wilkins MBA.
References
- 1.Talbot JCC, Aronson JK, Stephens MDB. Stephens’ detection and evaluation of adverse drug reactions: principles and practice. John Wiley & Sons; Chichester, West Sussex, UK: 2011. [Google Scholar]
- 2.Fung M. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets—1960 to 1999. Drug Inf J. 2001;35:293–317. [Google Scholar]
- 3.Piccini JP, Whellan DJ, Berridge BR, et al. Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the critical path initiative. Am Heart J. 2009;158:317–26. doi: 10.1016/j.ahj.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.FDA . Innovation or stagnation: challenge and opportunity on the critical path to new medical products. Food and Drug Administration, U.S. Department of Health and Human Services; Washington, DC: 2004. [Google Scholar]
- 5.Al-Khatib SM, Calkins H, Eloff BC, et al. Developing the safety of atrial fibrillation ablation registry initiative (safari) as a collaborative pan-stakeholder critical path registry model: a Cardiac Safety Research Consortium “incubator” think tank. Am Heart J. 2010;160:619–26. doi: 10.1016/j.ahj.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Mauri L, Kereiakes DJ, Normand SL, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–1041. 1041, e1031. doi: 10.1016/j.ahj.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Blankenship JC, et al. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer MA, Karweit JA, Cascade EF, et al. Practice patterns and outcomes of percutaneous coronary interventions in the United States: 1995 to 1997. Am J Cardiol. 2002;89:924–9. doi: 10.1016/s0002-9149(02)02240-3. [DOI] [PubMed] [Google Scholar]
- 10.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–5. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 11.Popma JJ, Satler LF, Pichard AD, et al. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88:1569–78. doi: 10.1161/01.cir.88.4.1569. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor WB, Anstrom KJ, Muhlbaier LH, et al. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol. 2000;36:723–30. doi: 10.1016/s0735-1097(00)00777-4. [DOI] [PubMed] [Google Scholar]
- 13.Buresly K, Eisenberg MJ, Zhang X, et al. Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction. Arch Intern Med. 2005;165:784–9. doi: 10.1001/archinte.165.7.784. [DOI] [PubMed] [Google Scholar]
- 14.Shehab N, Sperling LS, Kegler SR, et al. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med. 2010;170:1926–33. doi: 10.1001/archinternmed.2010.407. [DOI] [PubMed] [Google Scholar]
- 15.Rao SV, Cohen MG, Kandzari DE, et al. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187–95. doi: 10.1016/j.jacc.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:191–7. doi: 10.1016/j.jcin.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the acuity trial. J Am Coll Cardiol. 2007;49:1362–8. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Rao SV, O'Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Doyle BJ, Rihal CS, Gastineau DA, et al. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. 2009;53:2019–27. doi: 10.1016/j.jacc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 20.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–7. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–86. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Lansky AJ, Mehran R, Cristea E, et al. Impact of gender and antithrombin strategy on early and late clinical outcomes in patients with non–ST-elevation acute coronary syndromes (from the acuity trial). Am J Cardiol. 2009;103:1196–203. doi: 10.1016/j.amjcard.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Duvernoy CS, Smith DE, Manohar P, et al. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the blue cross blue shield of michigan cardiovascular consortium (bmc2) percutaneous coronary intervention registry. American Heart Journal. 2010;159:677–683. e671. doi: 10.1016/j.ahj.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–66. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 25.Alexander KP, Chen AY, Newby LK, et al. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–7. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 26.Eggebrecht H, von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol. 2004;16:247–50. [PubMed] [Google Scholar]
- 27.Tavris DR, Gallauresi BA, Lin B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol. 2004;16:459–64. [PubMed] [Google Scholar]
- 28.Ahmed B, Piper WD, Malenka D, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2:423–9. doi: 10.1161/CIRCINTERVENTIONS.109.860494. [DOI] [PubMed] [Google Scholar]
- 29.Peterson ED, Lansky AJ, Kramer J, et al. Effect of gender on the outcomes of contemporary percutaneous coronary intervention. Am J Cardiol. 2001;88:359–64. doi: 10.1016/s0002-9149(01)01679-4. [DOI] [PubMed] [Google Scholar]
- 30.Argulian E, Patel AD, Abramson JL, et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 31.Tizon-Marcos H, Bertrand OF, Rodes-Cabau J, et al. Impact of female gender and transradial coronary stenting with maximal antiplatelet therapy on bleeding and ischemic outcomes. Am Heart J. 2009;157:740–5. doi: 10.1016/j.ahj.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Marso SP, Amin AP, House JA, et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–64. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 33.Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–40. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–56. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 36.Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138:430–6. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 37.Chase AJ, Fretz EB, Warburton WP, et al. Association of the arterial access site at angioplasty with transfusion and mortality: the m.O.R.T.A.L study (Mortality Benefit of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008;94:1019–25. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- 38.Louvard Y, Lefevre T, Morice MC. Radial approach: what about the learning curve? Cathet Cardiovasc Diagn. 1997;42:467–8. doi: 10.1002/(sici)1097-0304(199712)42:4<467::aid-ccd30>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Mercuri M, Mehta S, Xie C, et al. Radial artery access as a predictor of increased radiation exposure during a diagnostic cardiac catheterization procedure. JACC Cardiovasc Interv. 2011;4:347–52. doi: 10.1016/j.jcin.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Louvard Y, Lefevre T, Allain A, et al. Coronary angiography through the radial or the femoral approach: the CARAFE study. Catheter Cardiovasc Interv. 2001;52:181–7. doi: 10.1002/1522-726x(200102)52:2<181::aid-ccd1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Ball WT, Sharieff W, Jolly SS, et al. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011;4:336–41. doi: 10.1161/CIRCINTERVENTIONS.110.960864. [DOI] [PubMed] [Google Scholar]
- 42.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 43.Barthelemy O, Silvain J, Brieger D, et al. Bleeding complications in primary percutaneous coronary intervention of ST-elevation myocardial infarction in a radial center. Catheter Cardiovasc Interv. 2012;79:104–12. doi: 10.1002/ccd.23164. [DOI] [PubMed] [Google Scholar]
- 44.Vorobcsuk A, Konyi A, Aradi D, et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction systematic overview and meta-analysis. Am Heart J. 2009;158:814–21. doi: 10.1016/j.ahj.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Joyal D, Bertrand OF, Rinfret S, et al. Meta-analysis of ten trials on the effectiveness of the radial versus the femoral approach in primary percutaneous coronary intervention. Am J Cardiol. 2012;109:813–8. doi: 10.1016/j.amjcard.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.06.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Pancholy S, Coppola J, Patel T, et al. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335–40. doi: 10.1002/ccd.21639. [DOI] [PubMed] [Google Scholar]