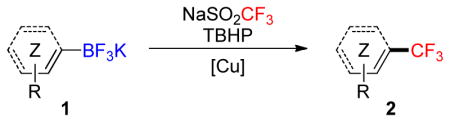
Table 1.
Scope of the Trifluoromethylation of Aryl- and Heteroaryltrifluoroboratesa
| ||||
|---|---|---|---|---|
| Entry | Substrate | Method a | Product | Yield b |
| 1 |
1a |
A |
2a |
99% (93%) |
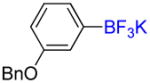
| 2 |
 1b |
A |
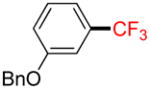 2b |
21% (14%) |
| 3 |
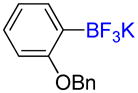 1c |
A |
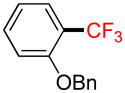 2c |
66% (50%) |
| 4 |
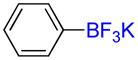 1d |
A |
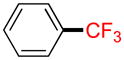 2d |
28% (−) |
| 5 |
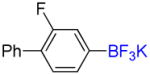 1e |
B |
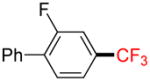 2e |
61% (34%) |
| 6 |
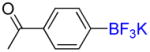 1f |
B |
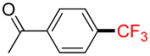 2f |
27% (−) |
| 7 |
1g |
A |
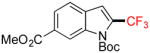 2g |
67% (64%) |
| 8 |
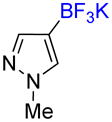 1h |
A |
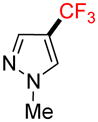 2h |
48% (−) |
| 9 |
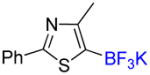 1i |
A |
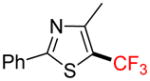 2i |
43% (24%) |
| 10 |
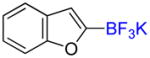 1j |
A |
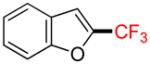 2j |
53% (−) |
| 11 |
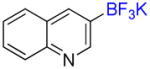 1k |
B |
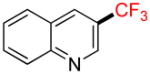 2k |
6% (−) |
Conditions A: NaSO2CF3 (3.0 equiv), TBHP (5.0 equiv), CuCl (1.0 equiv), CH2Cl2/MeOH/H2O, 1:1:0.8 ([1] = 0.1 M), open flask, rt, 12 h; conditions B: NaSO2CF3 (3.0 equiv), TBHP (4.0 equiv), (CH3CN)CuPF6 (1.0 equiv), NaHCO3 (1.0 equiv), MeOH ([1] = 0.1 M), open flask, rt, 12 h.
Yields determined by 19F analysis; isolated yields are reported in brackets.
