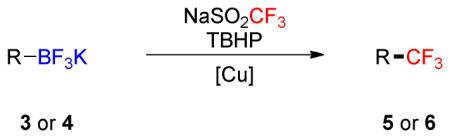
Table 2.
Scope of the Trifluoromethylation of Alkynyl- and Alkenyltrifluoroborates
| ||||
|---|---|---|---|---|
| Entry | Substrate | Method a | Product | Yield b |
| 1 |
3a |
A |
5a |
50% (−) |
| 2 |
3b |
A |
5b |
51% (45%) |
| 3 |
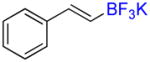 4a |
A |
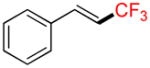 6a |
54% (−)c |
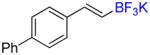
| 4 |
 4b |
A |
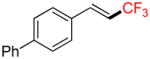 6b |
77% (77%)c |
| 5 |
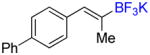 4c |
A |
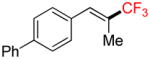 6c |
70% (59%) |
| 6 |
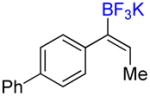 4d |
A |
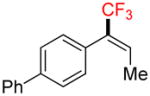 6d |
12% (10%) |
| 7 |
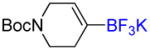 4e |
A | - | - |
Conditions A: NaSO2CF3 (3.0 equiv), TBHP (5.0 equiv), CuCl (1.0 equiv), CH2Cl2/MeOH/H2O, 1:1:0.8 ([3 or 4] = 0.1 M), open flask, rt, 12 h.
Yields determined by 19F analysis; isolated yields are reported in brackets.
With ~5% of the (Z) product.
