Scheme 1.
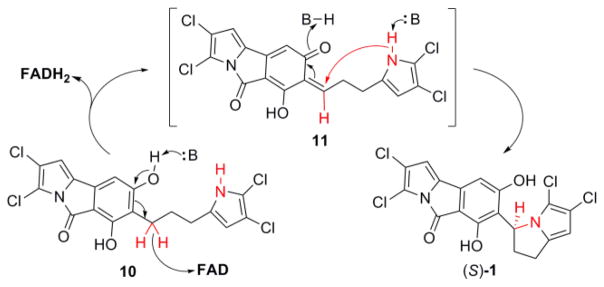
Proposed mechanisms for the stereoselective cyclization of 10 to (S)-1. Deprotonotation of the phenolic hydroxyl facilitates abstraction of the hydride by the FAD cofactor and generation of the intermediate 11. Further nucleophilic attack from the pyrrole-nitrogen yields (S)-1. The presence of molecular oxygen can restore the reduced FADH2 cofactor.
