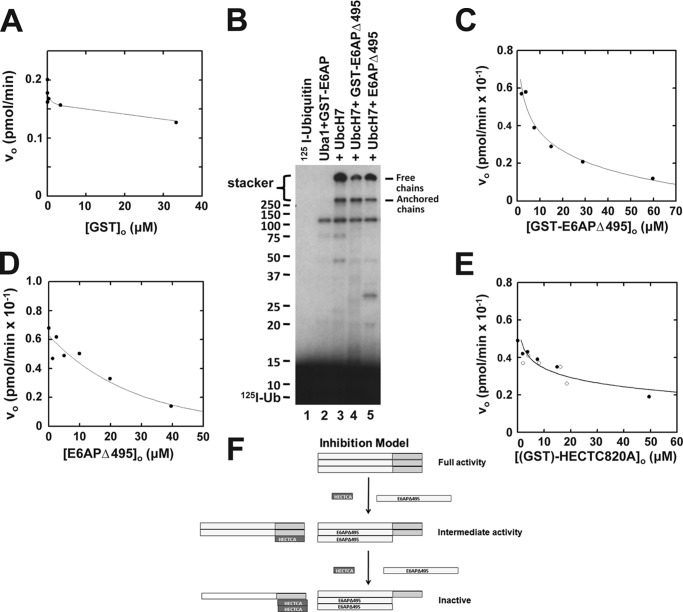
FIGURE 1.
E6AP catalyzed polyubiquitin chain formation requires oligomerization. A, initial rates of E6AP-catalyzed free polyubiquitin chain formation were determined as described under “Materials and Methods” in the presence of 8 nm human Uba1, 200 nm UbcH7, 0.2 nm GST-E6AP, 5 μm 125I-ubiquitin, and the indicated concentrations of recombinant free GST. B, autoradiogram of 125I-ubiquitin conjugation assays performed under initial velocity conditions in the presence of 60 nm human Uba1, 400 nm UbcH7, 6 nm GST-E6AP, 4 μm 125I-ubiquitin, and either 54 μm GST-E6APΔ495 or 66 μm E6APΔ495, as indicated. C, initial rates of 125I-ubiquitin conjugation were determined as in B in the presence of 8 nm GST-E6AP and the indicated concentrations of GST-E6APΔ495. As described under “Materials and Methods,” radioactivity associated with the stacker gel representing free and unanchored 125I-polyubiquitin chains was quantitated to calculate the resulting initial velocities. D, initial rates of 125I-ubiquitin conjugation were determined as in C but with the indicated concentrations of E6APΔ495. E, initial rate assays of 125I-ubiquitin conjugation determined as in C in the presence of the indicated concentrations of GST-HectC820A (open circles) or HectC820A (closed circles). F, schematic diagram depicting the model for inhibition of polyubiquitin chain formation by the Δ495 truncation or free Hect domain. For C–E, solid lines represent nonlinear inverse hyperbolic regression fits of the data using GraFit version 5.0.