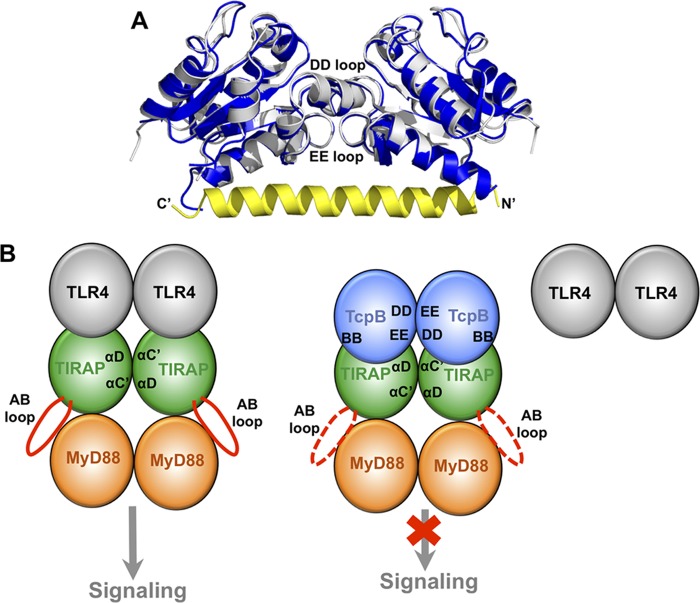
FIGURE 8.
The TcpB homodimer may mediate its association with TIRAP. A, comparison of the homodimeric TIR domain structures for the TcpBS124A/S127I mutant (blue) and the wild type (4LZP; silver). The N-terminal helical “tail” from the 4LZP structure is colored yellow with its N and C termini labeled. The DD and EE loops that mediate dimerization in both structures are marked. The view is rotated ∼90º horizontally from that in Fig. 4D. B, schematic representation of the TIRAP-mediated signaling complex formation. On the left, the TIRAP TIR domain (green) is recruited to the TLR4 TIR domain (gray), and a TIRAP TIR domain dimer formation mediated by its αC′ and αD helices exposes its AB loop (red) for recruitment of MyD88 (orange). The TcpB TIR domain (blue) forms homodimers through its DD and EE loops on the right. Upon association of TcpB with TIRAP, perhaps mediated by the BB loop of TcpB, TIRAP is no longer able to associate with TLR4 and induce downstream signaling.